Many readers are interested in the right subject, Icosapent Ethyl Capsules. Our manufacturer is pleased to report that we have already investigated contemporary research on the subject you are fascinated by. We will give you a wide range of answers based on the latest medical reports, advanced research papers, and sample surveys. Keep repeating for more information.
After oral administration, icosapent ethyl EPA is decorated during absorption and the active metabolite EPA is absorbed into the small intestine and into the systemic circulation via the thoracic lymphatic system. peak plasma concentrations of EPA were reached approximately 5 hours after oral administration. of icosapent ethyl .
ICOSAPENT Ethyl Capsules Prescribing Information
Drug Medical Testing. com. last updated on October 1, 2022.
On this page.
- Indications and Use
- Medication and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Side Effects
- Drug Interactions
- Use in specific populations
- Description
- Clinical Pharmacology
- Non-clinical toxicology
- Clinical Research
- Delivery/ storage and processing methods
- Information for Patients
ICOSAPENT Ethyl Capsule Indications and Dosing
Icosapent ethyl is indicated:
– In addition to diet to lower triglyceride (TG) levels in patients with difficult (≥500 mg/dl) hypertriglyceridemia full growth.
User Limitations
The effect of icosapent ethyl Risk of pancreatitis in patients with difficult hypertriglyceridemia has not been established.
Dosing and administration of ICOSAPENT Ethyl capsules
Before starting with Icosapent Ethyl.
– Evaluate lipid levels before starting therapy. Identify other causes of high triglyceride levels (diabetes, hypothyroidism or medications) and treat as needed. icosapent ethyl Patients should obtain proper diet and physiologic potency prior to receiving treatment. icosapent ethyl .
Medication and Administration
• The daily dose of icosapent ethyl 4 grams per day would be as follows: o 2 1 grams capsules Advise patient to swallow icosapent ethyl capsule completely. Do not break, crush, or chew the ethyl capsule.
Dosage Forms and Strengths
ICOSAPENT Ethyl Capsules are supplied in the form of 1 g, escape, elongated capsules with the product identification “54 648” on one side. capsule It has the product identification “54, 648” on one side.
Related/similar preparations
Contraindications
Icosapent ethyl Prohibited in patients with known hypersensitivity (e.g., anaphylactic reactions). to icosapent ethyl or one of its components.
Warnings and Precautions
Atrium Fibrillation/Atrium Flutter
Icosapent ethyl Associated with an increased risk of atrial fibrillation or atrial reversal requiring hospitalization, Atrium Fibrillation or Flutter was found in a double-blind, placebo-controlled study of 8, 179 subjects. icosapent ethyl The study compared 84 (2%) patients who received placebo [HR = 1, 5 (95% CI 1, 14, 1, 98). The incidence of atrial fibrillation was higher in patients with a history of atrial fibrillation or atrial fat.
Possible allergic reactions in patients with fish allergy
Icosapent ethyl contains ethyl Essential esters of the omega-3 fatty acid eicosapentanoic acid (EPA) from fish oil. It is not known whether patients with allergies to fish and/or shellfish are at increased risk for allergic reactions. to icosapent ethyl Inform patients about the possibility of allergic reactions in patients with known sensitivities to fish and shellfish to icosapent ethyl And advise them to stop icosapent ethyl And call for medical assistance if they respond.
Bleeding.
Icosapent ethyl Associated with an increased risk of bleeding: in a double-blind, placebo-controlled study of 8, 179 patients, 482 patients (12%) had an increased risk of bleeding. icosapent ethyl experience bleeding compared to 404 (10%) patients who received placebo; 111 (3%) patients experienced intolerable bleeding on icosapent ethyl 85 (2%) patients who received placebo. The incidence of bleeding was higher in patients who simultaneously received antithrombotic agents such as aspirin, clopidogrel, and warfarin.
Side Effects
The following important side effects are described below and in other labeling rooms
– Duplicate arrhythmia or atrium flutter [see Warnings and Precautions (5. 1)] – Possible allergic reaction in patients allergic to fish [see Warnings and Precautions (5. 2)] – Bleeding (see 5. 3) ).
Clinical experience control
Because clinical studies are conducted under very different circumstances, it is unlikely that the characteristics of adverse reactions observed in a clinical study of a product are directly comparable to and reflect the actual characteristics observed in a clinical study of another product.
Common side effects (incidence ≥3% and 1% more frequent). on icosapent ethyl and 1% more frequently than placebo) included musculoskeletal, peripheral edema, constipation, gout, and atrial fibrillation pain.
In two randomized, double-blind, placebo-controlled studies of patients with triglyceride levels between 200 and 2000 mg/dL treated for 12 months, adverse effects were reported more than 1% more frequently than placebo. icosapent ethyl Incidence was 1% more frequent than placebo based on pooled data including pulmonary portal disease and verbal pain.
Post-Marketing Experience
Additional adverse reactions were identified after approval of icosapent ethyl These reactions themselves have been reported by populations of indefinite size, so their frequency cannot usually be properly estimated or made causal to the effectiveness of the product.
– Diarrhea – Elevated blood triglycerides – Abdominal pain – Limb pain
Drug Interactions
Increased risk of bleeding with anticoagulants and anticoagulants.
Several studies using omega-3 fatty acids have shown prolongation of bleeding time. The prolonged bleeding times in these studies did not exceed the normal range and did not cause clinically significant bleeding. Patient Prognosis icosapent ethyl Concurrent anticoagulants and/or anticoagulants for bleeding.
Used in specific populations
Pregnancy
Available data from published case reports and drug surveillance databases on use of icosapent ethyl In pregnant women, there is little or no drug-related risk of maternal or fetal non-weight bearing birth defects, miscarriage, or non-dose-related risk of less favorable outcomes. In a reproductive study of pregnant rats, a non-dose-related imbalance for less significant developmental outcomes was observed during insertion into the oral cavity of icosapent ethyl During organogenesis of exposure equivalent to clinical effects at human doses of 4 g/day, based on body surface comparisons. In an oral study of pregnant rabbits. icosapent ethyl During organogenesis, based on body surface comparisons, one exposure greater than the clinical effect was clinically insignificant, not clinically significant (see data).
The estimated background risk of non-fatty birth defects and miscarriages in this population is unknown. All pregnancies have a background risk of birth defects, costs, or other adverse outcomes. In the general U.S. population, the estimated risk of major birth defects and miscarriages in clinically recognized pregnancies is 2-4% and 15-20%, accordingly.
In pregnant rats receiving oral digestive from 0, 3, 1, and 2 g/kg/day icosapent ethyl All groups matched to drug/day based on body surface from gestation to organogenesis.
In studies of multiple uses in pregnant rats shown 0, 3, 1, and 3 g/kg/day when used icosapent ethyl by oral administration through the stomach from Drachtag 7-17, icosapent ethyl had no effect on fetal viability (F1 or F2). non-dose-related imbalance in the results of optic nerve deficits and distorted testicular atrophy in human exposures based on body surface comparisons with the highest dose of 4 g/day. During the same exposure, a supplementary variant consisting of early incentive and enlargement of the cervical rib cervical percentage was observed. Pups from the highest dose mother animals showed reduced filling rate, delayed development, fewer implantations, and fewer viable fetuses (F2), possibly resulting in multiplexing. of icosapent ethyl In 7 human systemic exposures, the dose of 4 g/day based on a body surface per shape comparison.
In pregnant rabbits, oral stomach doses of 0, 1, 0, 3, and 1 g/kg/day icosapent ethyl After pregnancy until organogenesis, a decrease in body weight and food intake was observed at the highest dose of 1 g/kg/day (based on body comparisons, 5 times the effect of the highest dose on the highest dose on people was observed. surface). a slight increase in absorbed fetuses and dead fetuses was observed in the 1 g/kg/day group, but these were not significantly different from the control group. There were no differences between the icosapent ethyl group and the control group regarding the number of firms, the number of transplants, the number of viable fetuses, gender comparisons, the multiplicity of maternal fetal bodies or the authority of the placenta. Virtually all related to healing resulted in skeletal abnormalities or no skeletal abnormalities.
Given in pregnant rats. icosapent ethyl 0, 3, 1, and 3 g/kg/day from day 17 to day 20 of lactation, MAMMA effect or becoming was less favorable. In particular, an absolute loss of nests was seen in 2/23 nests at low doses. / day based on body surface comparisons.
Breastfeeding
In the published study, omega-3 fatty acids, including EPA, were found in breast milk. Women who received omega-3 fatty acids as a supplement received higher levels of omega-3 fatty acids than those who received omega-3 fatty acids in breast milk. No information is available on the effects of omega-3 fatty acids ethyl essential esters in infants during breastfeeding or milk production. The clinical needs of the mother and the clinical needs of the mother as well as the superior quality of the place and well field are footprints to be considered for icosapent ethyl And possible adverse effects on breastfeeding of the baby from the mother icosapent ethyl or from the mother’s primary mother.
Pediatrics
Safety and efficacy in pediatric patients is not incremental.
Geriatric
Of the total number of patients in a well-controlled clinical study of icosapent ethyl 45% were 65 years of age or older. No joint differences in safety or efficacy were observed between these patients and the larger juvenile group. Other enrolled clinical skills showed no difference in response between older and younger patients.
Liver Impairment
In patients with liver dysfunction, footsteps of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are occasionally checked during treatment. icosapent ethyl .
ICOSAPENT Ethyl – Capsule Description
Icosapent ethyl The lipid modulator is supplied in the form of 1 gram with a liquid-filled soft gelatin capsule capsule for oral administration.
Icosapent ethyl is an ethyl Ester of the omega-3 fatty acid eicosapentanoic acid (EPA). The empirical formula is. of icosapent ethyl C 22 H 34 O 2, and molecular groups are 330, 51. Chemical Name. for icosapent ethyl is ethyl All-CIS-5, 8, 11, 14, 17 Icosapentaenoate with correct chemical structure:
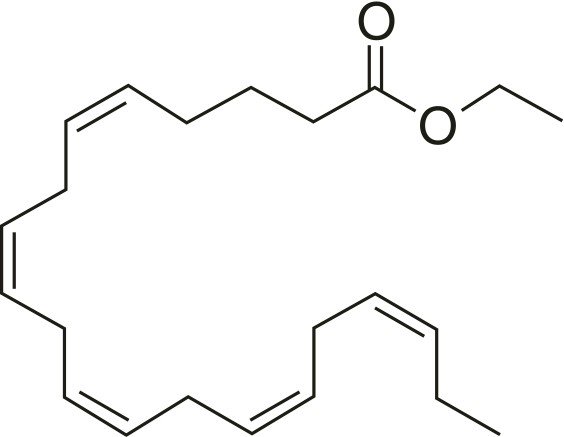
Each capsule It contains appropriate inert ingredients such as gelatin, glycerin, clear water, sorbitol, sorbitone, and tocopherol. Monogram ink ingredients include ammonium hydroxide, iron oxide, dark, isopropyl alcohol, macrogol, polyvinyl farate, propylene glycol, purified water, and SDA alcohol (ethanol). and ethyl acetate).
Icosapent Ethanol Capsules-Clinical Pharmacology
Mechanism.
Studies have shown that EPA decreases lipoprotein synthesis and/or secretion at fairly low densities (LLDL-TG) and increases the removal of Tg from circulating LLDL particles. Possible mechanisms of action include increased β-oxidation Inhibition of acyl CoA: 1,2-diacylglycerol acyltransferase (DGAT); decreased hepatic lipogenesis; enlarged plasma lipoprotein lipase output.
Pharmacodynamics
At 12-week dosing studies in patients with difficult hypertriglyceridemia, icosapent ethyl 4 g/day Median TG decreased compared to placebo [see Clinical Studies (14)].
Pharmacokinetics
After oral administration, icosapent ethyl EPA is decorated during absorption and the active metabolite EPA is absorbed into the small intestine and into the systemic circulation via the thoracic lymphatic system. peak plasma concentrations of EPA were reached approximately 5 hours after oral administration. of icosapent ethyl .
Icosapent ethyl administered with or after food in all clinical studies. Nutritional effects have not been investigated. Take icosapent ethyl with or after meals.
The average steady-state variance size of EPA is about 88 liters. The majority of EPA in plasma is integrated into phospholipids, triglycerides, and cholesteryl star, EPA is metabolized into the major metabolic
EPA is metabolized by the liver primarily through beta-oxidation, similar to fatty acids in food. Beta-oxidation breaks down the long carbon chain of EPA in the acetyl-Co enzyme A, which is converted to energy via the Krebs cycle. Cytochrome P450-membered metabolism is considered a less important method of EPA removal.
The total plasma report from steady state EPA is 684 ml/hour. the EPA plasma removal half value (T 1/2) is about 89 hours. icosapent ethyl Not subject to renal excretion.
When administered icosapent ethyl Clinical examination revealed no significant differences in plasma EPA concentrations between males and females of the weaker sex.
The pharmacokinetics of icosapent ethyl Not studied in pediatric patients.
Liver or renal dysfunction
Icosapent ethyl Not studied in patients with renal or hepatic dysfunction.
Drug Interactions
Omeprazole: In an interaction study with 28 awake adult subjects, icosapent ethyl 4 g/day of steady state omeprazole from a persistent AUCτ or C max is not quite correct when administered at a steady state exposure of 40 mg/day.
Rosiglitazone: in an interaction study with 28 awake adult subjects, icosapent ethyl 4 g/day from a steady-state 8 mg rosiglitazone single dose AUC or C max is not significant.
Warfarin: in a pharmacokinetic interaction study with 25 adult subjects in awareness, icosapent ethyl steady state 4 g/day of warfarin was administered as a single dose AUC or C-Max R and S-WARFARIN or 25 mg of Racic Warfarine simultaneously, adjusting for anticoagulant pharmacodynamics.
Atorvastatin: in a drug-pharmacology interaction study in 26 healthy adult subjects, icosapent ethyl A dose of 4 g/day of balance status altered the equilibrium AUCτ or Cmax status from atorvastatin, 2-hydroxyacid type blasts, or 4-hydroxyacid motor factor blasts, even with concurrent use of atorvastatin 80 mg/day in balance.
Non-clinical toxicology
Carcinogenesis, mutagenesis, fertility problems
In a 2-year carcinogenicity study in rats at oral doses of 0, 09, 0, 27, 0, 91 g/kg/day via probes. icosapent ethyl Thus, no neoplasms associated with the medical product pathway were detected in males. Hemangiomas and angiosarcomas in mesenteric lymph nodes were observed based on clinically significant exposures based on comparisons of body surfaces of various people with relatively high medical doses of 4 g/day, which is the absorption chamber of the product. The total incidence of hemangiomas and angiosarcomas in all vascular tissues was not increased in the healing background.
In a 6-month carcinogenicity study in transgenic mice of Tg. oral doses of Rash2 via probes of 0, 5, 1, 2, 4, 6 g/kg/kg. icosapent ethyl In male mice treated with the highest dose of the product, benign squamous papillomas fells were observed in the skin and subcutaneous tissue of the tail, which were related to the product pathway. It was assumed that the papillomas were not clinically significant as a result of acquired proximal tail irritation associated with fecal excretion of oil. No neoplasms associated with the product pathway were observed in female mice.
Icosapent ethyl Bacterial mutagens testing (AME) or trace testing in in vivo mice was not mutagenic with or without metabolic activation. Tests for chromosomal aberrations in Chinese hamster ovary (CHO) cells yielded flattering results for clastogenicity, with or without metabolic activation.
In an oral fertility study in rats, ethyl EPC was administered at doses of 0, 3, 1, and 3 kg/kg/day in male rats 9 months before the merger and female rats 14 days before gestation day 7. Female pups showed deuterogenic distance expansion and neck rib bone expansion observed at the 3 g/kg/day dose (7 times the systemic effect of the 4 g/day medical dose in people based on body surface comparisons) .
Clinical Research
Severe hypervolemia
The effects of icosapent ethyl In a randomized, placebo-controlled, double-blind study in a parallel group of adult patients, an intake of 4 g/day was observed (76 on icosapent ethyl , 75 on placebo) with severe hypertriglyceridemia. Patients whose baseline TG levels were between 500 and 2,000 mg/dL were enrolled in this study for 12 weeks. The median baseline TG and LDL-C levels in these patients were 684 mg/dL and 86 mg/dL, respectively. Median baseline HDL-C level was 27 mg/dL. The randomized population in this study was mostly Caucasian (88%) and male (76%). The mean age was 53 years and the mean body mass index was 31 kg/m 2 . Twenty-five percent of patients were on concomitant statin therapy, 28% were diabetics, and 39% of the patients had TG levels >750 mg/dl.
The most important lipoprotein pipid changes in the group icosapent ethyl received placebo or not are displayed in Table 2.
Table 2. median percent change in lipids compared to initial values in patients with difficult hypertriglyceridemia (≥500 mg/dl)
icosapent ethyl 4 g/day
ICOSAPENT Ethyl Capsule
Drug Testing with Drugs. com. last updated on August 28, 2022.
ICOSAPENT Ethyl capsule use:
- Used to lower triglycerides.
- Along with other cholesterol products, it is used to lower the risk of heart attacks, cardiac procedures, and chest pain (unstable angina).
- This can be given to you for other reasons. Discuss this with your doctor.
What should I tell my doctor before taking icosapent ethyl capsules?
- If you are allergic to this medicine. icosapent ethyl capsules ); all parts of this medicine ( icosapent ethyl capsules ); or another medicine, food, or medication. Tell your doctor about your allergy and the symptoms you have.
This medicine can interact with other medicines and health problems.
Tell your doctor and pharmacist about all your avenues (prescription or freely available medicines, natural products, vitamins) and health problems. You should make sure that it is safe for you to take this medicine ( icosapent ethyl capsules ) with all your medications and health problems. Do not start, stop, or change the dosage of your medicine without consulting your doctor.
what should i do if i use ethyl icosapenta capsules?
- Tell all your own medical staff that you are using this medication ( icosapent ethyl capsules ) This applies to doctors, nurses, pharmacists, and dentists.
- If you are allergic to fish, fish fat, or shellfish, talk to your own doctor.
- Have your blood checked as your doctor indicates for you. Consult your health care professional.
- Follow the diet and exercise program prescribed by your physician.
- Consult your physician before drinking alcohol.
- Certain types of abnormal heartbeats (atrial fibrillation or chest fluttering) have occurred with this drug ( icosapent ethyl capsules ) These abnormal heartbeats can be serious. The risk is increased in people who have had these abnormal heartbeats in the past.
- If you are pregnant, tell your doctor if you plan to become pregnant or breastfeed. You and your baby should have a conversation about the benefits and risks.
How is this medicine (ICOSAPENT Ethyl Capsule) taken?
Use this medicine ( icosapent ethyl capsules ) as prescribed by your doctor. Read all the information you receive. Pay attention to all instructions.
- Take this medicine ( icosapent ethyl capsules ) with food.
- Swallow this. Do not chew, break, open, or settle.
- If you have trouble swallowing, consult your own doctor.
- Keep this medicine ( icosapent ethyl capsules ) as your doctor or other care provider told you even if you feel better.
What should I do if I skip a dose?
- Take the missed dose as soon as you recognize it.
- If there is almost time for the proper dose, skip the missed dose and return to normal time.
- Do not take two doses or additional doses at the same time.
Should I report this to my doctor immediately?
WARNING/ WARNING: Despite the fact that it is rare, some people can experience fairly serious and sometimes fatal side effects when using this product. If you have any of the symptoms or signs that may be associated with serious side effects, tell your doctor or find medical help right away.
- Signs of an allergic reaction; skin rash, such as. Birdhouses; itching; reddish, swollen, blistered, or flaky skin with or without fever. Creaky breathing; stuffing in chest or throat. Difficulty breathing, swallowing, or talking. Unusual ho ho voice; or swelling of mouth, face, lips, tongue, or larynx.
- Fast or abnormal heartbeat.
- Dizziness or loss of consciousness.
- Shortness of breath.
- Chest pain.
- Unexplained bruising or bleeding.
- Swelling of the arms or legs.
What are the adverse effects of ICOSAPENT Ethyl Capsules?
All substances can cause side effects. However, many people have no or only mild side effects. Call your doctor or seek medical advice if you are suffering from any of these or other side effects, or if they do not go away.
- Muscle or joint pain.
- Constipation.
- Sore throat.
These are not all side effects that may occur. If you have questions about side effects, call your doctor. Ask your doctor about side effects.
You can report side effects to the FDA by calling 1-800-332-1088. https: / / You can also report side effects to www. FDA.Gov/Medwatch.
If an overdose is suspected:
If you suspect you have taken an overdose, call the anti-fifcentrum immediately or seek medical assistance. Be willing to tell or indicate what was taken, how much was taken, and when it occurred.
How should ICOSAPENT Ethyl Capsules be stored and/or discarded?
- Store in a dry space at room temperature. Do not store in the bathroom.
- Store all medications in a safe space. Keep all medications out of the reach of children and pets.
- Throw away unused or expired medications. Do not rinse the toilet and pour water down the drain unless you are told to do this. If you have questions about throwing away medications, talk to your pharmacist. Your area may have a medication return program.
Use of Customer Information and Disclaimer
- If symptoms or problems do not improve or worsen, call your doctor.
- Do not share your medications with others and do not take outside medications.
- For some medications, package leaflets are for patients. Talk to your pharmacist. If you have questions about this medication ( icosapent ethyl capsules ), consult your own physician, nurse, pharmacist or other care provider.
- If you suspect you have taken an overdose, call the anti-fifcentrum immediately or seek medical assistance. Be willing to tell or indicate what was taken, how much was taken, and when it occurred.
Frequently asked questions.
More about icosapent
- Review interactions
- Review (109)
- Pictures of medications
- Side effects
- Dosing Information
- Pregnancy
- Drug Class: Antiphopholipolytic of any kind
- In Spanish
Patient Source
- Medication Information
- Icosapent ethyl (Advanced Reading)
Other Brands
Specialty Sources
Related Treatments
- Hypervolemia
- Reducing Risk for Mental Health and Vascular Risk
For more information.
Always consult with your care provider to ensure that the information on this page is used in your case. & lt; Pran & gt;) speak with your own doctor, nurse, pharmacist, or other care provider.






