The results of use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), in treatment of onychomycosis of the toenail without lunula involvement were obtained from two double-blind, placebo-controlled studies conducted in the US. In these studies, patients with onychomycosis of the great toenails without lunula involvement were treated with ciclopirox topical solution, 8% in conjunction with monthly removal of the unattached, infected toenail by the investigator. Ciclopirox Topical Solution, 8%, (Nail Lacquer), was applied for 48 weeks. At baseline, patients had 20–65% involvement of the target great toenail plate. Statistical significance was demonstrated in one of two studies for the endpoint “complete cure” (clear nail and negative mycology), and in two studies for the endpoint “almost clear” (≤ 10% nail involvement and negative mycology) at the end of study. These results are presented below.
Ciclopirox Topical Solution, 8%
For use on fingernails and toenails and immediately adjacent skin only. Not for use in eyes.
DESCRIPTION
Ciclopirox Topical Solution, 8% contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin.
Each gram of Ciclopirox Topical Solution, 8% contains 80 mg ciclopirox in a solution base consisting of butyl ester of poly [vinylmethylether/maleic acid copolymer] in isopropyl alcohol, ethyl acetate, and isopropyl alcohol. Ethyl acetate and isopropyl alcohol are solvents that vaporize after application.
Ciclopirox Topical Solution, 8% is a clear, colorless to slightly yellowish solution.
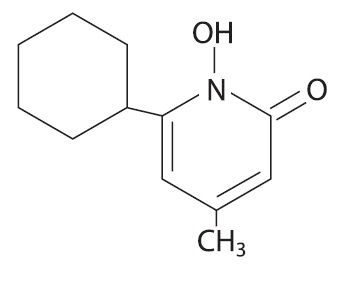
The chemical name for ciclopirox is 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone, with the empirical formula C 12 H 17 NO 2 and a molecular weight of 207.27.
The CAS Registry Number is [29342-05-0]. The chemical structure is:
CLINICAL PHARMACOLOGY
Microbiology
Mechanism of Action – The mechanism of action of ciclopirox has been investigated using various in vitro and in vivo infection models. One in vitro study suggested that ciclopirox acts by chelation of polyvalent cations (Fe +3 or Al +3 ) resulting in the inhibition of the metal-dependent enzymes that are responsible for the degradation of peroxides within the fungal cell. The clinical significance of this observation is not known.
Activity in vitro and ex vivo – In vitro methodologies employing various broth or solid media with and without additional nutrients have been utilized to determine ciclopirox minimum inhibitory concentration (MIC) values for the dermatophytic molds. (1-2) As a consequence, a broad range of MIC values, 1-20 ug/mL, were obtained for Trichophyton rubrum and Trichophyton mentagrophytes species. Correlation between in vitro MIC results and clinical outcome has yet to be established for ciclopirox.
One ex vivo study was conducted evaluating 8% ciclopirox against new and established Trichophyton rubrum and Trichophyton mentagrophytes infections in ovine hoof material. (3) After 10 days of treatment the growth of T. rubrum and T. mentagrophytes in the established infection model was very minimally affected. Elimination of the molds from hoof material was not achieved in either the new or established infection models.
Susceptibility testing for Trichophyton rubrum species – In vitro susceptibility testing methods for determining ciclopirox MIC values against the dermatophytic molds, including Trichophyton rubrum species, have not been standardized or validated. Ciclopirox MIC values will vary depending on the susceptibility testing method employed, composition and pH of media and the utilization of nutritional supplements. Breakpoints to determine whether clinical isolates of Trichophyton rubrum are susceptible or resistant to ciclopirox have not been established.
Resistance – Studies have not been conducted to evaluate drug resistance development in T. rubrum species exposed to 8% ciclopirox topical solution. Studies assessing cross-resistance to ciclopirox and other known antifungal agents have not been performed.
Antifungal Drug Interactions – No studies have been conducted to determine whether ciclopirox might reduce the effectiveness of systemic antifungal agents for onychomycosis. Therefore, the concomitant use of 8% ciclopirox topical solution and systemic antifungal agents for onychomycosis is not recommended.
Pharmacokinetics – As demonstrated in pharmacokinetic studies in animals and man, ciclopirox olamine is rapidly absorbed after oral administration and completely eliminated in all species via feces and urine. Most of the compound is excreted either unchanged or as glucuronide. After oral administration of 10 mg of radiolabeled drug (14C-ciclopirox) to healthy volunteers, approximately 96% of the radioactivity was excreted renally within 12 hours of administration. Ninety-four percent of the renally excreted radioactivity was in the form of glucuronides. Thus, glucuronidation is the main metabolic pathway of this compound.
Systemic absorption of ciclopirox was determined in five patients with dermatophytic onychomycoses, after application of ciclopirox topical solution, 8%, to all 20 digits and adjacent 5 mm of skin once daily for six months. Random serum concentrations and 24 hour urinary excretion of ciclopirox were determined at two weeks and at 1, 2, 4 and 6 months after initiation of treatment and four weeks post-treatment. In this study, ciclopirox serum levels ranged from 12-80 ng/mL. Based on urinary data, mean absorption of ciclopirox from the dosage form was
In two vehicle-controlled trials, patients applied ciclopirox topical solution, 8%, to all toenails and affected fingernails. Out of a total of 66 randomly selected patients on active treatment, 24 had detectable serum ciclopirox concentrations at some point during the dosing interval (range 10.0-24.6 ng/mL). It should be noted that 11 of these 24 patients took concomitant medication containing ciclopirox as ciclopirox olamine.
The penetration of the ciclopirox topical solution, 8% was evaluated in an in vitro investigation. Radiolabeled ciclopirox applied once to onychomycotic toenails that were avulsed demonstrated penetration up to a depth of approximately 0.4 mm. As expected, nail plate concentrations decreased as a function of nail depth. The clinical significance of these findings in nail plates is unknown. Nail bed concentrations were not determined.
INDICATIONS AND USAGE
(To understand fully the indication for this product, please read the entire INDICATIONS AND USAGE section of the labeling.)
Ciclopirox Topical Solution, 8% as a component of a comprehensive management program, is indicated as topical treatment in immunocompetent patients with mild to moderate onychomycosis of fingernails and toenails without lunula involvement, due to Trichophyton rubrum . The comprehensive management program includes removal of the unattached, infected nails as frequently as monthly, by a health care professional who has special competence in the diagnosis and treatment of nail disorders, including minor nail procedures.
• No studies have been conducted to determine whether ciclopirox might reduce the effectiveness of systemic antifungal agents for onychomycosis. Therefore, the concomitant use of 8% ciclopirox topical solution and systemic antifungal agents for onychomycosis, is not recommended. • Ciclopirox Topical Solution, 8% should be used only under medical supervision as described above. • The effectiveness and safety of ciclopirox topical solution, 8% in the following populations has not been studied. The clinical trials with use of ciclopirox topical solution, 8% excluded patients who: were pregnant or nursing, planned to become pregnant, had a history of immunosuppression (e.g., extensive, persistent, or unusual distribution of dermatomycoses, extensive seborrheic dermatitis, recent or recurring herpes zoster, or persistent herpes simplex), were HIV seropositive, received organ transplant, required medication to control epilepsy, were insulin dependent diabetics or had diabetic neuropathy. Patients with severe plantar (moccasin) tinea pedis were also excluded. • The safety and efficacy of using Ciclopirox Topical Solution, 8% daily for greater than 48 weeks have not been established.
Clinical Trials Data –
The results of use of ciclopirox topical solution, 8% in treatment of onychomycosis of the toenail without lunula involvement were obtained from two double-blind, placebo-controlled studies conducted in the United States. In these studies, patients with onychomycosis of the great toenails without lunula involvement were treated with ciclopirox topical solution, 8% in conjunction with monthly removal of the unattached, infected toenail by the investigator. Ciclopirox topical solution, 8%, was applied for 48 weeks. At baseline, patients had 20–65% involvement of the target great toenail plate. Statistical significance was demonstrated in one of two studies for the endpoint “complete cure” (clear nail and negative mycology), and in two studies for the endpoint “almost clear” (≤10% nail involvement and negative mycology) at the end of study. These results are presented below.
At Week 48 (plus Last Observation Carried Forward) for the Intent-to-Treat (ITT) Population
DESCRIPTION
Ciclopirox Topical Solution, 8%, (Nail Lacquer) contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin.
Each gram of Ciclopirox Topical Solution, 8%, (Nail Lacquer), contains 80 mg ciclopirox in a solution base consisting of ethyl acetate, NF; isopropyl alcohol, USP; and butyl monoester of poly[methylvinyl ether/maleic acid] in isopropyl alcohol. Ethyl acetate and isopropyl alcohol are solvents that vaporize after application.
Ciclopirox Topical Solution, 8%, (Nail Lacquer), is a clear, colorless to slightly yellowish solution.
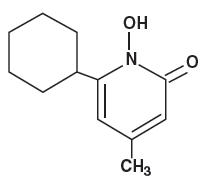
The chemical name for ciclopirox is 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone, with the molecular formula C 12 H 17 NO 2 and a molecular weight of 207.27. The CAS Registry Number is [29342-05-0]. The chemical structure is:
CLINICAL PHARMACOLOGY
Microbiology
Mechanism of Action
The mechanism of action of ciclopirox has been investigated using various in vitro and in vivo infection models. One in vitro study suggested that ciclopirox acts by chelation of polyvalent cations (Fe +3 or Al +3 ) resulting in the inhibition of the metal-dependent enzymes that are responsible for the degradation of peroxides within the fungal cell. The clinical significance of this observation is not known.
Activity in vitro and ex vivo
In vitro methodologies employing various broth or solid media with and without additional nutrients have been utilized to determine ciclopirox minimum inhibitory concentration (MIC) values for the dermatophytic molds. (1-2) As a consequence, a broad range of MIC values, 1-20 mcg/mL, were obtained for Trichophyton rubrum and Trichophyton mentagrophytes species. Correlation between in vitro MIC results and clinical outcome has yet to be established for ciclopirox.
One ex vivo study was conducted evaluating 8% ciclopirox against new and established Trichophyton rubrum and Trichophyton mentagrophytes infections in ovine hoof material. (3) After 10 days of treatment the growth of T. rubrum and T. mentagrophytes in the established infection model was very minimally affected. Elimination of the molds from hoof material was not achieved in either the new or established infection models.
Susceptibility testing for Trichophyton rubrum species
In vitro susceptibility testing methods for determining ciclopirox MIC values against the dermatophytic molds, including Trichophyton rubrum species, have not been standardized or validated. Ciclopirox MIC values will vary depending on the susceptibility testing method employed, composition and pH of media and the utilization of nutritional supplements. Breakpoints to determine whether clinical isolates of Trichophyton rubrum are susceptible or resistant to ciclopirox have not been established.
Resistance
Studies have not been conducted to evaluate drug resistance development in T. rubrum species exposed to 8% ciclopirox topical solution. Studies assessing cross-resistance to ciclopirox and other known antifungal agents have not been performed.
Antifungal Drug Interactions
No studies have been conducted to determine whether ciclopirox might reduce the effectiveness of systemic antifungal agents for onychomycosis. Therefore, the concomitant use of 8% ciclopirox topical solution and systemic antifungal agents for onychomycosis is not recommended.
Pharmacokinetics
As demonstrated in pharmacokinetic studies in animals and man, ciclopirox olamine is rapidly absorbed after oral administration and completely eliminated in all species via feces and urine. Most of the compound is excreted either unchanged or as glucuronide. After oral administration of 10 mg of radiolabeled drug (14C-ciclopirox) to healthy volunteers, approximately 96% of the radioactivity was excreted renally within 12 hours of administration. Ninety-four percent of the renally excreted radioactivity was in the form of glucuronides. Thus, glucuronidation is the main metabolic pathway of this compound.
Systemic absorption of ciclopirox was determined in 5 patients with dermatophytic onychomycoses, after application of Ciclopirox Topical Solution, 8%, (Nail Lacquer), to all 20 digits and adjacent 5 mm of skin once daily for six months. Random serum concentrations and 24 hour urinary excretion of ciclopirox were determined at two weeks and at 1, 2, 4 and 6 months after initiation of treatment and 4 weeks post-treatment. In this study, ciclopirox serum levels ranged from 12-80 ng/mL. Based on urinary data, mean absorption of ciclopirox from the dosage form was
In two vehicle-controlled trials, patients applied Ciclopirox Topical Solution, 8%, (Nail Lacquer), to all toenails and affected fingernails. Out of a total of 66 randomly selected patients on active treatment, 24 had detectable serum ciclopirox concentrations at some point during the dosing interval (range 10.0-24.6 ng/mL). It should be noted that eleven of these 24 patients took concomitant medication containing ciclopirox as ciclopirox olamine cream, 0.77%.
The penetration of the Ciclopirox Topical Solution, 8%, (Nail Lacquer), was evaluated in an in vitro investigation. Radiolabeled ciclopirox applied once to onychomycotic toenails that were avulsed demonstrated penetration up to a depth of approximately 0.4 mm. As expected, nail plate concentrations decreased as a function of nail depth. The clinical significance of these findings in nail plates is unknown. Nail bed concentrations were not determined.
INDICATIONS AND USAGE
(To understand fully the indication for this product, please read the entire INDICATIONS AND USAGE section of the labeling.)
Ciclopirox Topical Solution, 8%, (Nail Lacquer), as a component of a comprehensive management program, is indicated as topical treatment in immunocompetent patients with mild to moderate onychomycosis of fingernails and toenails without lunula involvement, due to Trichophyton rubrum . The comprehensive management program includes removal of the unattached, infected nails as frequently as monthly, by a health care professional who has special competence in the diagnosis and treatment of nail disorders, including minor nail procedures.
- No studies have been conducted to determine whether ciclopirox might reduce the effectiveness of systemic antifungal agents for onychomycosis. Therefore, the concomitant use of 8% ciclopirox topical solution and systemic antifungal agents for onychomycosis, is not recommended.
- Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be used only under medical supervision as described above.
- The effectiveness and safety of Ciclopirox Topical Solution, 8%, (Nail Lacquer), in the following populations has not been studied. The clinical trials with use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), excluded patients who: were pregnant or nursing, planned to become pregnant, had a history of immunosuppression (e.g., extensive, persistent, or unusual distribution of dermatomycoses, extensive seborrheic dermatitis, recent or recurring herpes zoster, or persistent herpes simplex), were HIV seropositive, received organ transplant, required medication to control epilepsy, were insulin dependent diabetics or had diabetic neuropathy. Patients with severe plantar (moccasin) tinea pedis were also excluded.
- The safety and efficacy of using Ciclopirox Topical Solution, 8%, (Nail Lacquer), daily for greater than 48 weeks have not been established.
Clinical Trials Data
The results of use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), in treatment of onychomycosis of the toenail without lunula involvement were obtained from two double-blind, placebo-controlled studies conducted in the US. In these studies, patients with onychomycosis of the great toenails without lunula involvement were treated with ciclopirox topical solution, 8% in conjunction with monthly removal of the unattached, infected toenail by the investigator. Ciclopirox Topical Solution, 8%, (Nail Lacquer), was applied for 48 weeks. At baseline, patients had 20–65% involvement of the target great toenail plate. Statistical significance was demonstrated in one of two studies for the endpoint “complete cure” (clear nail and negative mycology), and in two studies for the endpoint “almost clear” (≤ 10% nail involvement and negative mycology) at the end of study. These results are presented below.
| Study 312 | Study 313 | |||
|---|---|---|---|---|
| Active | Vehicle | Active | Vehicle | |
| * Clear nail and negative mycology † ≤ 10% nail involvement and negative mycology ‡ Negative KOH and negative culture | ||||
| Complete Cure * | 6/110 (5.5%) |
1/109 (0.9%) |
10/118 (8.5%) |
0/117 (0%) |
| Almost Clear † | 7/107 (6.5%) |
1/108 (0.9%) |
14/116 (12%) |
1/115 (0.9%) |
| Negative Mycology Alone ‡ | 30/105 (29%) |
12/106 (11%) |
41/115 (36%) |
10/114 (9%) |
The summary of reported patient outcomes for the ITT population at 12 weeks following the end of treatment are presented below. Note that post-treatment efficacy assessments were scheduled only for patients who achieved a complete cure.
| Study 312 | Study 313 | |||
|---|---|---|---|---|
| Active | Vehicle | Active | Vehicle | |
| * Four patients (from studies 312 and 313) who were completely cured did not have post-treatment Week 12 planimetry data. | ||||
| Number of Treated Patients | 112 | 111 | 119 | 118 |
| Complete Cure at Week 48 | 6 | 1 | 10 | 0 |
| Post-treatment Week 12 Outcomes: | ||||
| Patients Missing All Week 12 Assessments | 2 | 0 | 2 | 0 |
| Patients with Week 12 Assessments | 4 | 1 | 8 | 0 |
| Complete Cure | 3 | 1 | 4 | 0 |
| Almost Clear | 2* | 1 | 1* | 0 |
| Negative Mycology | 3 | 1 | 5 | 0 |
CONTRAINDICATIONS
Ciclopirox Topical Solution, 8%, (Nail Lacquer), is contraindicated in individuals who have shown hypersensitivity to any of its components.
WARNINGS
Ciclopirox Topical Solution, 8%, (Nail Lacquer), is not for ophthalmic, oral, or intravaginal use. For use on nails and immediately adjacent skin only.
PRECAUTIONS
If a reaction suggesting sensitivity or chemical irritation should occur with the use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), treatment should be discontinued and appropriate therapy instituted.
So far there is no relevant clinical experience with patients with insulin dependent diabetes or who have diabetic neuropathy. The risk of removal of the unattached, infected nail, by the health care professional and trimming by the patient should be carefully considered before prescribing to patients with a history of insulin dependent diabetes mellitus or diabetic neuropathy.
Information for Patients
Patients should have detailed instructions regarding the use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), as a component of a comprehensive management program for onychomycosis in order to achieve maximum benefit with the use of this product.
The patient should be told to:
- Use Ciclopirox Topical Solution, 8%, (Nail Lacquer), as directed by a health care professional. Avoid contact with the eyes and mucous membranes. Contact with skin other than skin immediately surrounding the treated nail(s) should be avoided. Ciclopirox Topical Solution, 8%, (Nail Lacquer), is for external use only.
- Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be applied evenly over the entire nail plate and 5 mm of surrounding skin. If possible, Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be applied to the nail bed, hyponychium, and the under surface of the nail plate when it is free of the nail bed (e.g., onycholysis). Contact with the surrounding skin may produce mild, transient irritation (redness).
- Removal of the unattached, infected nail, as frequently as monthly, by a health care professional is needed with use of this medication. Inform a health care professional if they have diabetes or problems with numbness in your toes or fingers for consideration of the appropriate nail management program.
- Inform a health care professional if the area of application shows signs of increased irritation (redness, itching, burning, blistering, swelling, oozing).
- Up to 48 weeks of daily applications with Ciclopirox Topical Solution, 8%, (Nail Lacquer), and professional removal of the unattached, infected nail, as frequently as monthly, are considered the full treatment needed to achieve a clear or almost clear nail (defined as 10% or less residual nail involvement).
- Six months of therapy with professional removal of the unattached, infected nail may be required before initial improvement of symptoms is noticed.
- A completely clear nail may not be achieved with use of this medication. In clinical studies less than 12% of patients were able to achieve either a completely clear or almost clear toenail.
- Do not use the medication for any disorder other than that for which it is prescribed.
- Do not use nail polish or other nail cosmetic products on the treated nails.
- Avoid use near heat or open flame, because product is flammable.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity study was conducted with Ciclopirox Topical Solution, 8%, (Nail Lacquer), formulation. A carcinogenicity study of ciclopirox (1% and 5% solutions in polyethylene glycol 400) in female mice dosed topically twice per week for 50 weeks followed by a 6-month drug-free observation period prior to necropsy revealed no evidence of tumors at the application sites.
In human systemic tolerability studies following daily application (~340 mg of Ciclopirox Topical Solution, 8%, (Nail Lacquer)) in subjects with distal subungual onychomycosis, the average maximal serum level of ciclopirox was 31±28 ng/mL after two months of once daily applications. This level was 159 times lower than the lowest toxic dose and 115 times lower than the highest nontoxic dose in rats and dogs fed 7.7 and 23.1 mg ciclopirox (as ciclopirox olamine)/kg/day.
The following in vitro genotoxicity tests have been conducted with ciclopirox: evaluation of gene mutation in Ames Salmonella and E. coli assays (negative); chromosome aberration assays in V79 Chinese hamster lung fibroblasts, with and without metabolic activation (positive); gene mutation assay in the HGPRT-test with V79 Chinese hamster lung fibroblasts (negative); unscheduled DNA synthesis in human A549 cells (negative); and BALB/c3T3 cell transformation assay (negative). In an in vivo Chinese hamster bone marrow cytogenetic assay, ciclopirox was negative for chromosome aberrations at 5,000 mg/kg.
The following in vitro genotoxicity tests were conducted with Ciclopirox Topical Solution, 8%, (Nail Lacquer): Ames Salmonella test (negative); unscheduled DNA synthesis in the rat hepatocytes (negative); cell transformation assay in BALB/c3T3 cell assay (positive). The positive response of the lacquer formulation in the BALB/c3T3 test was attributed to its butyl monoester of poly [methylvinyl ether/maleic acid] resin component (Gantrez ® ES-435), which also tested positive in this test. The cell transformation assay may have been confounded because of the film-forming nature of the resin. Gantrez® ES-435 tested nonmutagenic in both the in vitro mouse lymphoma forward mutation assay with or without activation and unscheduled DNA synthesis assay in rat hepatocytes.
Oral reproduction studies in rats at doses up to 3.85 mg ciclopirox (as ciclopirox olamine)/kg/day [equivalent to approximately 1.4 times the potential exposure at the maximum recommended human topical dose (MRHTD)] did not reveal any specific effects on fertility or other reproductive parameters. MRHTD (mg/m2) is based on the assumption of 100% systemic absorption of 27.12 mg ciclopirox (~340 mg Ciclopirox Topical Solution, 8%, (Nail Lacquer)) that will cover all the fingernails and toenails including 5 mm proximal and lateral fold area plus onycholysis to a maximal extent of 50%.
Pregnancy
Teratogenic effects
Pregnancy Category B
Teratology studies in mice, rats, rabbits, and monkeys at oral doses of up to 77, 23, 23, or 38.5 mg, respectively, of ciclopirox as ciclopirox olamine/kg/day (14, 8, 17, and 28 times MRHTD), or in rats and rabbits receiving topical doses of up to 92.4 and 77 mg/kg/day, respectively (33 and 55 times MRHTD), did not indicate any significant fetal malformations.
There are no adequate or well-controlled studies of topically applied ciclopirox in pregnant women. Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Since many drugs are excreted in human milk, caution should be exercised when Ciclopirox Topical Solution, 8%, (Nail Lacquer), is administered to a nursing woman.
Pediatric Use
Based on the safety profile in adults, Ciclopirox Topical Solution, 8%, (Nail Lacquer), is considered safe for use in children twelve years and older. No clinical trials have been conducted in the pediatric population.
Geriatric Use
Clinical studies of Ciclopirox Topical Solution, 8%, (Nail Lacquer), did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients.
ADVERSE REACTIONS
In the vehicle-controlled clinical trials conducted in the US, 9% (30/327) of patients treated with Ciclopirox Topical Solution, 8%, (Nail Lacquer), and 7% (23/328) of patients treated with vehicle reported treatment-emergent adverse events (TEAE) considered by the investigator to be causally related to the test material.
The incidence of these adverse events, within each body system, was similar between the treatment groups except for Skin and Appendages: 8% (27/327) and 4% (14/328) of subjects in the ciclopirox and vehicle groups reported at least one adverse event, respectively. The most common were rash-related adverse events: periungual erythema and erythema of the proximal nail fold were reported more frequently in patients treated with Ciclopirox Topical Solution, 8%, (Nail Lacquer), (5% [16/327]) than in patients treated with vehicle (1% [3/328]). Other TEAEs thought to be causally related included nail disorders such as shape change, irritation, ingrown toenail, and discoloration.
The incidence of nail disorders was similar between the treatment groups (2% [6/327] in the Ciclopirox Topical Solution, 8%, (Nail Lacquer), group and 2% [7/328] in the vehicle group). Moreover, application site reactions and/or burning of the skin occurred in 1% of patients treated with Ciclopirox Topical Solution, 8%, (Nail Lacquer), (3/327) and vehicle (4/328).
A 21-Day Cumulative Irritancy study was conducted under conditions of semi-occlusion. Mild reactions were seen in 46% of patients with the Ciclopirox Topical Solution, 8%, (Nail Lacquer), 32% with the vehicle and 2% with the negative control, but all were reactions of mild transient erythema. There was no evidence of allergic contact sensitization for either the Ciclopirox Topical Solution, 8%, (Nail Lacquer), or the vehicle base. In a separate study of the photosensitization potential of Ciclopirox Topical Solution, 8%, (Nail Lacquer), in a maximized test design that included the occluded application of sodium lauryl sulfate, no photoallergic reactions were noted. In four subjects localized allergic contact reactions were observed. In the vehicle-controlled studies, one patient treated with Ciclopirox Topical Solution, 8%, (Nail Lacquer), discontinued treatment due to a rash, localized to the palm (causal relation to test material undetermined).
Use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), for 48 additional weeks was evaluated in an open-label extension study conducted in patients previously treated in the vehicle-controlled studies. Three percent (9/281) of subjects treated with Ciclopirox Topical Solution, 8%, (Nail Lacquer), experienced at least one TEAE that the investigator thought was causally related to the test material. Mild rash in the form of periungual erythema (1% [2/281]) and nail disorders (1% [4/281]) were the most frequently reported. Four patients discontinued because of TEAEs. Two of the four had events considered to be related to test material: one patient’s great toenail “broke away” and another had an elevated creatine phosphokinase level on Day 1 (after 48 weeks of treatment with vehicle in the previous vehicle-controlled study).
To report SUSPECTED ADVERSE REACTIONS, contact G&W Laboratories, Inc. at 1-800-922-1038 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
DOSAGE AND ADMINISTRATION
Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be used as a component of a comprehensive management program for onychomycosis. Removal of the unattached, infected nail, as frequently as monthly, by a health care professional, weekly trimming by the patient, and daily application of the medication are all integral parts of this therapy. Careful consideration of the appropriate nail management program should be given to patients with diabetes (see PRECAUTIONS ).
Nail Care By Health Care Professionals
Removal of the unattached, infected nail, as frequently as monthly, trimming of onycholytic nail, and filing of excess horny material should be performed by professionals trained in treatment of nail disorders.
Nail Care By Patient
Patients should file away (with emery board) loose nail material and trim nails, as required, or as directed by the health care professional, every seven days after Ciclopirox Topical Solution, 8%, (Nail Lacquer), is removed with alcohol.
Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be applied once daily (preferably at bedtime or eight hours before washing) to all affected nails with the applicator brush provided. The Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be applied evenly over the entire nail plate.
If possible, Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be applied to the nail bed, hyponychium, and the under surface of the nail plate when it is free of the nail bed (e.g., onycholysis).
The Ciclopirox Topical Solution, 8%, (Nail Lacquer), should not be removed on a daily basis. Daily applications should be made over the previous coat and removed with alcohol every seven days. This cycle should be repeated throughout the duration of therapy.
HOW SUPPLIED
Ciclopirox Topical Solution, 8%, (Nail Lacquer), is supplied in 6.6 mL (NDC 51672-5302-0) glass bottles with screw caps which are fitted with brushes.
Protect from light (e.g., store the bottle in the carton after every use).
Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be stored at room temperature between 59° and 86° F (15° and 30° C).
CAUTION: Flammable. Keep away from heat and flame.
Rx ONLY
References
1. Dittmar W., Lohaus G. 1973. HOE296, A new antimycotic compound with a broad antimicrobial spectrum. Arzneim-Forsch./Drug Res. 23:670-674.
2. Niewerth et. al., 1998. Antimicrobial susceptibility testing of dermatophytes: Comparison of the agar macrodilution and broth micro dilution tests. Chemotherapy. 44:31-35.
3. Yang et. al. 1997. A new simulation model for studying in vitro topical penetration of antifungal drugs into hard keratin. J. Mycol. Med. 7:195-98.
Gantrez is a registered trademark of GAF Corporation
Mfd. by:
Cosette Pharmaceuticals, Inc.
111 Coolidge Street, South Plainfield, NJ 07080
Dist. by:
Taro Pharmaceuticals U.S.A., Inc.
Hawthorne, NY 10532
8-0317TR03
Rev. 10/2019
Ciclopirox Topical Solution, 8%, (Nail Lacquer)
Patient Information and Instructions
Patients should have detailed instructions regarding the use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), as a component of a comprehensive management program for onychomycosis in order to achieve maximum benefit with the use of this product. Discuss your treatment plan with your health care professional for regular removal of the unattached, infected nail.
Before using this medication, tell your doctor if you:
- Are pregnant or nursing
- Are an insulin dependent diabetic or have diabetic neuropathy
- Have a history of immunosuppression
- Are immunocompromised (e.g., received an organ transplant, etc.)
- Require medication to control epilepsy
- Use or require topical corticosteroids on a repeated monthly basis
- Use steroid inhalers on a regular basis
Mfd. by:
Cosette Pharmaceuticals, Inc.
111 Coolidge Street, South Plainfield, NJ 07080
Dist. by:
Taro Pharmaceuticals U.S.A., Inc.
Hawthorne, NY 10532
8-0317TR03
Rev. 10/2019
PRINCIPAL DISPLAY PANEL – 6.6 mL Bottle Carton
Ciclopirox
Topical Solution
8%
(Nail Lacquer)
FOR DERMATOLOGIC
USE ONLY.
NOT FOR USE IN EYES.
Penlac (Ciclopirox) – Topical
Patricia Weiser, PharmD, is a licensed pharmacist and freelance medical writer.
Published on December 01, 2022
Mary Choy, PharmD, is board-certified in geriatric pharmacotherapy and is an active leader in professional pharmacy associations.
Additional Content by IBM Micromedex ®
Table of Contents
Table of Contents
What Is Penlac?
Penlac (ciclopirox) is a prescription-only nail lacquer, which comes as a topical liquid solution that you apply directly to your nails. A topical medication is applied directly to a particular place on the body. Penlac is used to treat fungal nail infections, also called onychomycosis.
Ciclopirox is an antifungal drug. Specifically, it belongs to a drug class called hydroxypyridone antimycotics. Penlac works by penetrating deep into the layers of the nail bed and surrounding skin. It kills fungus by blocking fungus from getting the nutrients it needs to grow.
Drug Facts
Generic Name: Ciclopirox
Brand Name: Penlac
Drug Availability: Prescription
Administration Route: Topical
Therapeutic Classification: Hydroxypyridone antimycotic
Available Generically: Yes
Controlled Substance: No
Active Ingredient: Ciclopirox
Dosage Form(s): Laquer, cream, shampoo
What Is Penlac Used For?
Penlac treats mild to moderate fungal nail infections, also called onychomycosis, caused by the fungus Trichophyton rubrum. This fungus is the most common cause of fungal nail infections.
Fungal nail infections are common in toenails but can also occur in fingernails. Fingernails or toenails with a fungal infection often appear discolored (usually yellow, brown, or white), thick, or cracked.
Anyone can get a fungal nail infection, but the following populations may be more likely than others to get a fungal nail infection:
- People with diabetes or poor circulation
- People with a weakened immune system (such as HIV or other immune system problems)
- People who often get athlete’s foot (a fungal skin infection of the toes/foot)
How to Use Penlac
Penlac Nail Lacquer is applied to the infected nail once a day, preferably at bedtime, according to the steps below:
- If the infected nail has any loose areas, use a nail file or clippers to remove the loose nail material before applying Penlac.
- Use the included applicator brush to apply Penlac in an even layer over the infected nail. Be sure to coat your nail bed and the underside of the nail. Avoid getting Penlac on your skin, except for the skin that borders the infected nail.
- After applying the solution, give it a minute to dry before covering the nails with socks or gloves. Make sure to wait eight hours before taking a shower or bath.
The next day, apply another layer of ciclopirox over the previous coat. After a week of daily treatment, remove the nail lacquer with rubbing alcohol. Then return to the first step.
If you cannot trim your nail, you will likely need to have a caregiver or healthcare provider trim the infected nails once a month or as directed while using Penlac. Talk to your healthcare provider before trimming your nails if you have diabetes or problems with numbness in your hands or feet.
Here are a few additional tips to keep in mind when using Penlac:
- Be careful not to get Penlac in your eyes, mouth, or genital area.
- Do not use nail polish or other nail cosmetics.
- Avoid using ciclopirox nail solution near an open flame because it is flammable. If you smoke, wait until the product is completely dry.
Storage
Penlac Nail Lacquer comes in a glass bottle with a screw-on cap with an attached applicator brush. Store the bottle in its original carton (to protect it from light) at room temperature between 59 and 86 degrees Fahrenheit (15 and 30 degrees Celsius). The product is flammable, so keep it from heat or open flame. Do not store it in the bathroom.
Close the bottle tightly after each use to prevent the solution from drying. It is also essential to prevent the solution from getting into the bottle threads, or else the cap may get stuck.
How Long Does Penlac Take to Work?
It takes a long time to treat fungal infections of the nails. However, most people notice an improvement within six months of daily Penlac use.
Fungal nail infections can be stubborn. As a result, Penlac may not completely clear the infection. Among people who used Penlac in a 48-week clinical trial for toenail fungal infections, less than 12% of people achieved clear or nearly clear results.
What Are the Side Effects of Penlac?
This is not a complete list of side effects, and others may occur. A healthcare provider can advise you on side effects. If you experience other effects, contact your pharmacist or healthcare provider. You may report side effects to the Food and Drug Administration (FDA) at fda.gov/medwatch or 800-FDA-1088.
Common Side Effects
Common side effects of using Penlac include:
- Temporary skin irritation: Mild redness or burning sensation may occur where the solution comes in contact with your skin. Do your best to avoid getting Penlac Nail Lacquer on your skin, except for skin that borders your infected nail.
- Nail discoloration
- Change in nail shape
- Ingrown toenail
Severe Side Effects
Penlac should not cause serious side effects, but severe skin reactions in application areas have been reported. Tell your healthcare provider if you notice severe symptoms such as:
- Severe irritation of the nail area: Oozing, blistering, burning, itching, or reddening
- Allergic reaction: Rash
Long-Term Side Effects
Penlac is not known to cause long-term side effects.
Report Side Effects
Penlac may cause other side effects. Call your healthcare provider if you have any unusual problems while taking this medication.
If you experience a serious side effect, you or your healthcare provider may send a report to the FDA’s MedWatch Adverse Event Reporting Program or by phone (800-332-1088).
Dosage: How Much Penlac Should I Use?
Drug Content Provided and Reviewed by IBM Micromedex ®
The dose of this medicine will be different for different patients. Follow your doctor’s orders or the directions on the label. The following information includes only the average doses of this medicine. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
- For topical cream and lotion dosage forms:
- Fungus infections (treatment):
- Adults and children 10 years of age and over—Apply two times a day, morning and evening.
- Children up to 10 years of age—Use and dose must be determined by your doctor.
- Fungus infections (treatment) or seborrheic dermatitis (treatment):
- Adults and children 16 years of age and over—Apply two times a day, morning and evening.
- Children up to 16 years of age—Use and dose must be determined by your doctor.
- Seborrheic dermatitis (treatment):
- Adults and children 16 years of age and over—Apply 1 teaspoon (or up to 2 teaspoons for long hair) two times a week for four weeks with at least three days between each application.
- Children up to 16 years of age—Use and dose must be determined by your doctor.
- Fungus infections (treatment):
- Adults —Apply once daily, preferably at bedtime or eight hours before washing.
- Children up to 18 years of age—Use and dose must be determined by your doctor.
Modifications
The following modifications (changes) should be kept in mind when using Penlac:
Severe allergic reaction: Avoid using Penlac if you have a known allergy to it or its ingredients. Ask your pharmacist or healthcare provider for a complete list of the ingredients if you’re unsure.
Missed Dose
If you miss an application of Penlac, continue using it the next day at your usual time. Do not apply extra to make up for a missed dose.
Penlac treats a fungal nail infection best when you use it consistently. Try setting an alarm or reminder on your phone to help you remember to use it daily.
Overdose: What Happens If I Take Too Much Penlac?
There is limited information available about a Penlac overdose.
If you think you’re experiencing an overdose or life-threatening symptoms, seek immediate medical attention.
What Happens If I Overdose on Penlac?
If you think you or someone else may have overdosed on Penlac, call a healthcare provider or the Poison Control Center (800-222-1222).
If someone collapses or isn’t breathing after taking Penlac, call 911 immediately.
Precautions
Drug Content Provided and Reviewed by IBM Micromedex ®
If your skin problem does not improve within 2 to 4 weeks, or if it becomes worse, check with your doctor.
Inform your doctor right away if the area where you applied the medicine shows signs of increased irritation (e.g., redness, itching, burning, blistering, swelling, or oozing) because it could be an allergic reaction.
Nail problems treated with the topical solution form of this medicine may take up to 6 months to start improving.
To help clear up your infection completely and to help make sure it does not return, good health habits are also required. The following measures will help reduce chafing and irritation and will also help keep the area cool and dry.
- For patients using ciclopirox for ringworm of the groin (tinea cruris):
- Avoid wearing underwear that is tight-fitting or made from synthetic materials (for example, rayon or nylon). Instead, wear loose-fitting, cotton underwear.
- Use a bland, absorbent powder (for example, talcum powder) or an antifungal powder (for example, tolnaftate) on the skin. It is best to use the powder between applications of ciclopirox.
- Carefully dry the feet, especially between the toes, after bathing.
- Avoid wearing socks made from wool or synthetic materials (for example, rayon or nylon). Instead, wear clean, cotton socks and change them daily or more often if the feet sweat freely.
- Wear sandals or well-ventilated shoes (for example, shoes with holes on top or on the side).
- Use a bland, absorbent powder (for example, talcum powder) or an antifungal powder (for example, tolnaftate) between the toes, on the feet, and in socks and shoes freely once or twice a day. It is best to use the powder between applications of ciclopirox.
If you have any questions about these measures, check with your health care professional.
What Are Reasons I Shouldn’t Use Penlac?
You should not use Penlac if you have had an allergic reaction to ciclopirox or any of its ingredients.
What Other Medications Interact With Penlac?
Drug interactions occur when one drug affects how another drug works. No specific medicines are known to interact with Penlac.
Penlac Nail Lacquer is a topical solution. It is applied directly to the infected nail—the drug works within the layers of the nail bed and surrounding skin, killing fungus. Very little of the drug absorbs into your bloodstream. Therefore, it is unlikely to interfere with other medications.
Before using Penlac, it is still a good idea to tell your healthcare provider about your current medications, including prescription drugs, over-the-counter (OTC) medicines, vitamins, herbs, and other dietary supplements. Sharing this information can help to prevent potentially harmful drug interactions.
What Medications Are Similar?
Penlac contains the active ingredient ciclopirox. It is a unique antifungal drug that you apply to the infected nail. Other antifungal medicines may be prescribed to treat fungal infections, but they do not work precisely the same way as ciclopirox.
Some examples of other antifungal drugs are:
- Lamisil (terbinafine) – oral: Oral terbinafine (prescription-only) can be effective but may take several months to work. Some potential downsides include getting blood work and side effects such as headache, upset stomach, and liver problems.
- OTC antifungals – topical: Some examples include Fungi-Nail and Kerasal. They may take several months to work and may not work for everyone.
- Jublia (efinaconazole) – topical: Jublia is a prescription-only topical antifungal. It is similar to Penlac in that you paint it directly on the infected toenail, which may take up to 48 weeks to work.
Ask your pharmacist or a healthcare provider if you have questions about alternatives to Penlac.
Frequently Asked Questions
What is Penlac used for?
Penlac is prescribed to treat nail fungal infections in which nails become discolored (usually yellow or white), thick, and prone to breakage. Penlac is applied directly to the infected nail once daily.
Does Penlac have a generic version?
Yes, a generic version of the drug is available: ciclopirox 8% topical solution. Generic medicines tend to cost less than brand-name medications.
Can I paint my nails after using Penlac?
You should not apply nail polish or other nail cosmetics, such as artificial nails, to the infected nail during your treatment with Penlac. Using nail polish or fake nails will prevent the medication from penetrating deep into the layers of your nail, where it works to kill the infection-causing fungus.
How well does Penlac work?
Most people notice improvement while using Penlac. But your nail may not look clearer until you have used this treatment consistently for several months. Fungal nail infections can take a long time to treat, and it is possible that Penlac may not completely clear the infection. Less than 12% of people who used Penlac in a clinical trial achieved clear or nearly clear results after about 11 months (48 weeks) of treatment.
How Can I Stay Healthy While Taking Penlac?
Fungal infections can discolor your nails and may make some people feel self-conscious. The good news is that several treatment options are available, including Penlac Nail Lacquer. Keep in mind that nail fungal infections, while common, can be stubborn, and it often takes several months of treatment to see improvement. Not everyone achieves completely clear nails even after 48 weeks (about 11 months) of treatment with Penlac.
If you are not seeing improvement within six months, consider talking to your healthcare provider about other treatment options.
Medical Disclaimer
Verywell Health’s drug information is meant for educational purposes only and is not intended as a replacement for medical advice, diagnosis, or treatment from a healthcare provider. Consult your healthcare provider before taking any new medication(s). IBM Watson Micromedex provides some of the drug content, as indicated on the page.
Verywell Health uses only high-quality sources, including peer-reviewed studies, to support the facts within our articles. Read our editorial process to learn more about how we fact-check and keep our content accurate, reliable, and trustworthy.
- Food and Drug Administration. Penlac nail lacquer (ciclopirox) topical solution, 8%.
- Centers for Disease Control and Prevention. Fungal nail infections.
- Food and Drug Administration. Jublia (efinaconazole) topical solution.
By Patricia Weiser, PharmD
Patricia Weiser, PharmD, is a licensed pharmacist and freelance medical writer. She has more than 14 years of professional experience.
- Fungus infections (treatment):






