Many readers are interested in the appropriate topic: ammonium lactate cream, 12%*. We are pleased to report that our author has already researched the latest studies on your fascinating topic. We offer a wide range of answers based on the latest medical reports, advanced research papers, and sample surveys. Please repeat for further study.
Ammonium Lactate Cream 12% is indicated for the treatment of ichthyosis vulgaris and psoriasis.
Information on Ammonium Lactate Cream
Medical tests evaluated by drug. Com. Last updated on September 1, 2021.
On this page.
- Description.
- Clinical Pharmacology
- Indications and Uses
- Contraindications
- Warnings
- Precautions
- Patient Support Information
- Side Effects
- Medication and Administration
- Shipping, Storage and Handling
For dermatological use only.
Do not use for ophthalmic, oral, or vaginal applications.
Description of Ammonium Lactate Cream
*Ammonium lactate cream 12% formula 12% lactic Acid neutralized ammonium hydroxide, as ammonium lactate pH 4.4 to 5.4. ammonium creme, 12% contains cetyl alcohol, glycerin, glyceryl stearate, laureth-4, nonheavy mineral oil, magnesium aluminum silicate, methylcellulose, methylparaben, PEG-100 stearate, polyoxyl stearate 40, propylene glycol, propylparaben, clear water.
Lactic acid produces a racemic mixture of 2-hydroxypropanoic acid and contains the correct structural formula.
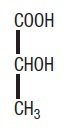
Ammonium Lactate Cream – Clinical Pharmacology
Lactic acid produces alpha-hydroxy acids. It is a common element in tissues and blood. Alpha hydroxy acids (and their salts) have been found to act as moisturizers when applied to the skin. This property has the ability to affect the hydration of the stratum corneum. Apart from this, lactic acid is applied to the skin, it may decrease the adhesive strength of the stratum corneum. Devices to achieve this are not yet widespread.
A study of transdermal absorption of ammonium lactate cream inserted into the skin of a human cadaver showed that approximately 6.1% of the substance was absorbed after 68 hours.
Indications and Usage of Ammonium Lactate Cream
Ammonium Lactate Cream 12% is indicated for the treatment of ichthyosis vulgaris and psoriasis.
Related and Similar Drugs
Contraindications
Cremat – Ammonium Lactate Cream, 12% is contraindicated in patients with a history of hypersensitivity to any of the ingredients listed on the label.
Warning.
The effects of the sun on Ammonium 12% Cream skin should be minimized or avoided (see Precautions). If hypersensitivity to any of the components is determined, use of this product should be discontinued.
Precautions
General – For external use.
For external use only. If the product is distorted (e.g., after shaving legs), which is canyoned, eroded, or distorted in an offensive manner, drops or burning may occur when the product is applied to the skin. Caution should be exercised when applying to the face due to the potential for irritation. The potential for post-inflammatory hypo or hyperpigmentation has not been investigated.
Patient Information
Patients using ammonium lactate 12% should receive appropriate information and instructions.
1. this medication should be used as directed by a physician and may not be used for conditions different from those for which it is prescribed. It is for external use only. Be careful of contact with eyes, sponges, or mucous membranes.2. Patients should minimize or ignore the use of this product on any part of the skin that will likely be exposed to natural or artificial sunlight by covering that person. If the effects of the sun are unavoidable, clothing should be worn to protect the skin; 3. this drink may cause a burning sensation when applied to skin with cracks, erosions, or abrasions (e.g., after shaving legs); 4. if the skin condition worsens during treatment, the medication should be immediately should be stopped.
carcinogenesis, mutagenesis, or birth defects.
The 12%, 21%, or 30% ammonium lactate 2-year direction composition did not result in a significant increase in skin tumors or systemic tumors when the effects of UV light were not available. The maximum systemic effect in mice in this study was 0.7 times the highest possible systemic effect in humans. However, a long-term study of photocarcinogenicity in Albinowitte mice showed that 12% ammonium lactate ammonium lactate was applied locally and the rate of tumor formation induced by UV light.
Possible mutants of ammonium lactate were investigated by the AMES test and the in vivo mocrinecleus test in mice. Both were negative.
In skin sections I and III, studies using ammonium lactate cream at dose levels of 300 mg/kg/day (1800 mg/m 2/day) in rats with pre or repeated parameters, about 0, 4 times in rats, virtually no effect was observed in pre or repeated parameters No effect was observed. District doses for people.
Pregnancy:
Teratogenic Effects:
Pregnancy Category B-.
There have been animal reproduction studies in rats and rabbits with corresponding human doses (600 mg/kg/day 5x, 7200 mg, 3600 mg/m corresponding to 5x, 7, and 1 // 2/day in rabbits) and there is little indication in these to reduce fetal fertility or damage by ammonium lactate. However, there are no adequate and completely controlled studies for pregnant women. Animal reproductive studies do not always predict human response, so ammonium lactate may be used only when clearly necessary.
Breastfeeding mothers – acid is considered a normal part of the blood.
Although lactic Although acid is considered a normal component of blood and tissue, it is not clear to what extent this product affects the normal acid content of breast milk. lactic Acid levels in breast milk. Because almost all drugs are different in breast milk, caution should be exercised when crematory lactamonium is administered to a breastfeeding woman.
Pediatric Use
The safety and efficacy of ammonium lactamonium cream in a 2-year-old patient was 12%.
Geriatric Applications.
Clinical studies of ammonium lactate cream have determined whether 12% do not include the required number of subjects over 65 years of age and therefore respond differently than younger subjects. Another clinical skill reported showed no difference in response between older and younger patients. In general, dose selection should be done with caution in older patients.
Adverse Effects
In a controlled clinical study of patients with mastopathy, the most common side effects in patients treated with ammonium lactate were rash (including erythema and irritation) and burning/biting. Both were reported in approximately 10-15% of patients. When not included, Plytas was reported in about 5% of patients.
In a controlled clinical study of Xerosis patients, the most unusual side effects were burning sensation of ammonium lactate, treated in about 3% of patients, stooping, dry skin, and skin rash, each reported in about 2% of patients.
Ammonium lactate should be administered and
Apply to affected areas and massage gently; use twice daily or as prescribed by a physician.
How is ammonium lactate supplied?
Ammonium lactate cream, 12% is available as follows
385 g bottle (NDC 45802-49 3-26)
Two tubes of 280 g (NDC 45802- 49 3-83) Boxes of 140 g (NDC 45802-49 3-98)
Storage.
Save at 20-25°C (68-77°F) [see USP control room temperature].
Manufactured by Perrigo
Perrigo®Allegan, MI 4901 0-www. Perrigo. Cartoon
Pavement Main Display
ndc 45802- 493-83.
Ammonium lactate cream, 12%.
For dermatological use only.
Do not use for ophthalmic, oral, or vaginal applications.

The following image is a placeholder that suggests a personal product number to be applied or printed on the label with the medical product during the packaging process.

| Product Information | |||
| Type | Medical product label regarding a person’s prescription | Dust Code (source) | NDC: 45802-493 |
| Management | Related | DEA Schedule | |
| Active Component/ Active Component | ||
| Name Component | Strength based on | Courage |
| Ammonium lactate (lactic acid) | Lactic acid | 12 g in 100 g |
| Inactive Ingredients | |
| Name Component | Courage |
| Cetyl alcohol | |
| Glycerol | |
| Glyceryl monostrate | |
| Light mineral oil | |
| Magnesium hard film silicate | |
| Methylparaben | |
| PEG-100 stearate | |
| Polyoxyl 40 stearate | |
| Propylene glycol | |
| Propylpara | |
| Water | |
| Lorette 4 | |
| Package | |||
| # | Product Code | Packaging Description | |
| 1 | NDC: 45802-493-83 | 2 tubes in 1 box | |
| 1 | 140 g in 1 tube | ||
| 2 | NDC: 45802-493-26 | 385 g in 1 bottle, pump | |
| 3 | NDC: 45802-493-98 | 140 g in 1 tube | |
| Marketing Information | |||
| Marketing Category | Order number or quoted monograph | Start Date Marketing | End date marketing |
| and a | ANDA075774 | 16. 08. 2006 | |
| Lean Car – Padagis Israel Pharmaceuticals Ltd (600093611) |
Padagis Israel Pharmaceutics Ltd
More about ammonium lactate topical
- Compare Alternatives
- Prices & Coupons
- Reviews (17)
- Side Effects
- Dosing Information
- Pregnancy
- Medication class: Urgent Moderation
- and español
Patient Source
- Medication Information
- Lactic Acid and Ammonium Hydroxide – Ammonium Cream
- Lactic Acid and Ammonium Hydroxide Lotion
Professional Resources
Description
*Ammonium lactate cream 12% formula 12% lactic Acid neutralized ammonium hydroxide, as ammonium lactate pH 4.4 to 5.4. ammonium creme, 12% contains cetyl alcohol, glycerin, glyceryl stearate, laureth-4, nonheavy mineral oil, magnesium aluminum silicate, methylcellulose, methylparaben, PEG-100 stearate, polyoxyl stearate 40, propylene glycol, propylparaben, clear water.
Lactic acid produces a racemic mixture of 2-hydroxypropanoic acid and contains the correct structural formula.

Clinical Pharmacology
Lactic acid produces alpha-hydroxy acids. It is a common element in tissues and blood. Alpha hydroxy acids (and their salts) have been found to act as moisturizers when applied to the skin. This property has the ability to affect the hydration of the stratum corneum. Apart from this, lactic acid is applied to the skin, it may decrease the adhesive strength of the stratum corneum. Devices to achieve this are not yet widespread.
In vitro studies on transdermal absorption of ammonium lactate cream use on human cadaver skin shows that after 68 hours approximately 6.1% of the material has been absorbed.
Indications and Uses
Ammonium Lactate Cream 12% is indicated for the treatment of ichthyosis vulgaris and psoriasis.
Contraindications.
Cremat – Ammonium Lactate Cream, 12% is contraindicated in patients with a history of hypersensitivity to any of the ingredients listed on the label.
Warning.
The effects of the sun on Ammonium 12% Cream skin should be minimized or avoided (see Precautions). If hypersensitivity to any of the components is determined, use of this product should be discontinued.
Precautionary Measures
General – For external use.
For external use only. If the product is distorted (e.g., after shaving legs), which is canyoned, eroded, or distorted in an offensive manner, drops or burning may occur when the product is applied to the skin. Caution should be exercised when applying to the face due to the potential for irritation. The potential for post-inflammatory hypo or hyperpigmentation has not been investigated.
Patient Information
Patients using ammonium lactate 12% should receive appropriate information and instructions.
1. this medication should be used as directed by a physician and may not be used for conditions different from those for which it is prescribed. It is for external use only. Be careful of contact with eyes, sponges, or mucous membranes.2. Patients should minimize or ignore the use of this product on any part of the skin that will likely be exposed to natural or artificial sunlight by covering that person. If the effects of the sun are unavoidable, clothing should be worn to protect the skin; 3. this drink may cause a burning sensation when applied to skin with cracks, erosions, or abrasions (e.g., after shaving legs); 4. if the skin condition worsens during treatment, the medication should be immediately should be stopped.
carcinogenesis, mutagenesis, or birth defects.
The 12%, 21%, or 30% ammonium lactate composition of the 2-year direction did not result in a significant increase in skin tumors or systemic tumors when the effects of UV light were not available. The maximum systemic effect in mice in this study was 0.7 times the highest possible systemic effect in humans. Nevertheless, long-term studies of photocarcinogenicity in Albinowitte mice have shown that the use of topically 12% ammonium lactate cream Increased rate of UV-induced skin tumorigenesis.
Mutagenicity. of ammonium lactate cream Evaluated by AMES test and in vivo Micrunecleus test in mice, both negative.
In skin section I and III studies ammonium lactate cream Little or no effect was observed on prenatal or postnatal development in rats at dose levels of 300 mg/kg/day (1800 mg/m 2/day), approximately 0.4 times the human dose.
Pregnancy:
Teratogenic Effects:
Pregnancy Category B-.
Animal and rabbit reproduction studies in rats are at corresponding human doses (600 mg/kg/day, 5-fold higher dose), corresponding to 3600 mg/m in rats EN 7200 mg. // 2/day in rabbits) and these gave little indication for reducing fetal birth rate or injury as a result of such effects. to ammonium lactate cream There are no adequate and fully controlled studies on pregnant women. Because animal reproduction studies do not always predict people’s responses, ammonium lactate cream may be used during pregnancy only when clearly necessary.
Breastfeeding mothers – acid is considered a normal part of the blood.
Although lactic Although acid is considered a normal component of blood and tissue, it is not clear to what extent this product affects the normal acid content of breast milk. lactic Acid content in breast milk. Almost all medications are different in breast milk and therefore attention is paid when footprints appear ammonium lactate cream operated by copper women.
Pediatric Use
The safety and efficacy of ammonium lactamonium cream in a 2-year-old patient was 12%.
Geriatric Applications.
Clinical studies of ammonium lactate cream The 12% did not include the required number of described subjects 65 years and older to determine if they responded differently than younger subjects. Another clinical skill reported showed no difference in response between older and younger patients. In general, dose selection should be done with caution in older patients.
Adverse Effects
In controlled clinical studies of patients with pregnancy syndrome, the most common side effects in treated patients were ammonium lactate cream skin rash (including erythema and irritation) and burning sensation/puncture. Both were reported in approximately 10-15% of patients. When not included, Plytas was reported in about 5% of patients.
In a controlled clinical study of Xerosis patients, the more unusual side effects in treated patients ammonium lactate cream penetrated about 3% of patients, penetrating puncture wounds, dry skin, and skin rashes. One of these was reported in about 2% of patients.
Medication and Administration
Apply to affected areas and massage gently; use twice daily or as prescribed by a physician.
How supplied.
Ammonium lactate cream, 12% is available as follows
385 g bottle (NDC 45802-49 3-26)
Two tubes of 280 g (NDC 45802- 49 3-83) Boxes of 140 g (NDC 45802-49 3-98)
Storage.
Save at 20-25°C (68-77°F) [see USP control room temperature].
Manufactured by Perrigo
Perrigo®Allegan, MI 4901 0-www. Perrigo. Cartoon
Pavement Main Display
ndc 45802- 493-83.
Ammonium lactate cream, 12%.
For dermatological use only.
Do not use for ophthalmic, oral, or vaginal applications.

The following image is a placeholder that suggests a personal product number to be applied or printed on the label with the medical product during the packaging process.






