Many readers are interested in the right subject: the side effects of Vityy. Our makers are pleased to report that they have already done modern research studies on your fascinating subject. We can give you a wide range of answers based on the latest medical reports, advanced research papers, and sample surveys. Keep repeating it to verify the details.
Aging does not occur in the pediatric population.
Vuity Eye Doples Refrance Information
Drug Medical Testing. com. last updated on November 1, 2022.
On this page.
- Indications and Use
- Medication and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Side Effects
- Use in specific populations
- Overdose
- Description
- Clinical Pharmacology
- Non-clinical toxicology
- Clinical Research
- Delivery/Storage and Treatment Methods
- Patient Information
1 indications and uses
Vitality is indicated for the healing of geriatric healing.
2 Dosage and administration
Recommended Dose Bithiol one drop Once daily in all eyes.
Apply more than one timely ophthalmic product, product is applied in 5 minute phases.
3 Dosages and Strengths
Vitality (Pilocarpine Hydrochloride Chloride Solution) is available as 1, 25% solution (12, 5 mg/ml).
4 Contraindications
Vigor is contraindicated in one patient with known hypersensitivity to the functional component or in patients led to its expression.
5 warnings and precautions
5. 1 Blurred vision.
Myotica has the opportunity to cause blurred vision in its dose of Vitualt. Patients are advised not to drive or operate machines if their vision is not clear (e.g., blurred vision).
In addition, patients have the opportunity to experience temporary dim or dark vision in Vitualt-covered myositis. Patients should be advised to be cautious of nighttime driving and other unsafe activities in reduced lighting conditions.
5. 2 Risk of retinal subsegmentation
Rare cases of retinal and rare cases of retinal rupture have been reported in myxomatosis and cover vorti
People with pre-existing retinal disease are at increased risk. This is why a retinal exam is recommended for all patients before treatment begins.
Patients are advised to seek medical assistance immediately with sudden flashing lights, floaters, or loss of face.
5, 3 Iritis.
Not recommended for use with iritis due to possible adhesions (synechiae) between the iris and lens.
5. 4 Use with contact lenses.
Contact lens wearers should be advised to remove the lens before administering Vuithy and after 10 minutes of administration before reinserting the contact lens.
5. 5 Potential for Eye Injury or Infection
To prevent eye injury or contamination, the dosing bottle should not come in contact with the eyes or other levels.
6 Adverse Reactions
The following clinically significant side effects are described in other rooms of the label
- Hypersensitivity [see Contraindications (4)].
6. 1 Clinical experience control
Because clinical studies are conducted under very different circumstances, it is unlikely that the characteristics of an adverse effect observed in a clinical study of a product are directly comparable to and reflect the actual characteristics of a clinical study of another product. In the case of the
VUITY was evaluated in 375 patients with presbyopia in two randomized, double-masked, vehicle-controlled studies (GEMINI 1 and GEMINI 2) of 30 days duration. The most common adverse reactions reported in >Headache and conjunctival congestion were present in 5% of patients. Ocular side effects reported in 1-5% of patients were vision, eye pain, visual disturbances, eye irritation, and weight loss of enlarged baumag.
6. 2 Email Marketing Skills.
The following adverse reactions were identified during the subsequent application of Vigor. Because these reactions are self-reported from indefinitely sized populations, it is not always possible to estimate their frequency or make a causal link to the effects of Vigor.
Eye disorders: glass moisture branches, vitreous microscopic involvement, retinal cracks, retinal subsegmentation.
8 Applicable to specific populations
8. 1 Pregnancy
Risk Overview. There are no adequate and well-controlled studies on the administration of Vuithy in pregnant women that provide information on the risks of the drug. No oral administration of pilocarpine to pregnant rats during organogenesis and lactation has caused adverse health effects at clinically relevant doses. effects At clinically relevant doses.
Human data. Pregnant women do not have adequate and good control checks from vitualts. In a retrospective case series of 15 women with glaucoma, 4 patients used ophthalmic pilocarpine before pregnancy, during pregnancy, and after delivery. There were no substantial adverse effects observed in the patients or their babies.
Animal data in embryogenesis studies, oral administration of pilocarpine to pregnant rats during organogenesis – Maternal toxicity, skeletal abnormalities, and fetal weight loss of 90 mg/kg/day (approximately 970 higher than the maximum or algal muscular photometry, one fetal weight loss []] 0, 015 mg/kg/day, mg/m2 base).
Ambient/postnatal examination in rats showed that oral administration of pilocarpine during late gestation resulted in more births at a dose of 36 mg/kg/day (about 390 more than MRHOD). Decreased neonatal survival and decreased average pup weight were observed at doses above 18 mg/kg/day (about 200 above the daily dose recommended for humans).
8. 2 Lactation
Risk Summary No information is available on the availability of pilocarpine in breast milk, the effects about breastfed babies, or the effects For milk production, estimate the risk of vitality in breastfed infants.
Pilocarpine and/or its metabolites are excreted in the milk of lactating rats. Systemic values for topical ocular administration of pilocarpine Na are low [see Clinical Pharmacology (12. 3)], and it is unknown whether measurable values of pilocarpine in breast milk occur after regional ocular administration.
Excellent qualities for the development and breastfeeding wells must be considered against the medical needs and adverse effects of maternal of Vuity. effects Breastfeeding by Vuity.
Animal data. after the first oral administration of 14 C-pillar pins to lactating rats, the concentration of radioactivity in milk was comparable to that in plasma.
8.4 Pediatric applications
Aging does not occur in the pediatric population.
8. 5 Geriatric use.
In the Vity clinical study, subjects over 65 years of age were not involved to determine if they responded differently than younger subjects; other reported clinical experiences with Pilocarpine Ophthalmic mixture showed no cumulative difference in protection between older and younger patients.
10 Overdose.
Systemic toxicity after topical use of pilocarpine in purification is rare, but sometimes sensitive patients may develop sweat ated intestinal hyperactivity. Accidental ingestion can cause sweating, salivation, nausea, tremors, wrist retardation, and decreased blood pressure. If moderate overdose occurs, spontaneous recovery, facilitated by intravenous water to compensate for dehydration, should be awaited. Patients with slow bowel infections should use atropine, a pharmacologic antagonist of pilocarpine.
11 Description.
VUITI (hydrochloride hydrochloride solution) 1, 25% is a cholinergic muscarinic receptor prepared as an isotonic dull sterile ophthalmic solution containing 1, 25% pilocarpine hydrochloride. The chemical name of pilocarpine hydrochloride is (3S, 4R)-3-ethyl-4-[(1-methyl-1H-imidazol-yl)methyl]oxalan-2- hydrochloride. The molecular formula is 244, 72 and the molecular formula is C 11 H 16 N 2 O 2-HCl. The structural formula is as follows
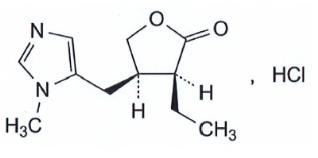
Each ML vuity contains 1.25% pilocarpine hydrochloride (12, 5 mg) as the concentrated component corresponding to 1.06% (10, 6 mg) free performance component. Storage method: benzalkonium chloride 0.0075%. The inactive components of the ocular solution are likely to be boric acid, sodium citrate dihydrate, sodium chloride, clear water, and hydrochloric acid and/or sodium hydroxide added to bring the pH to values of 3, 5, 5, 5 5, 5, 5 as needed.
12 Clinical Pharmacology
12.1 Mechanism of Action
Saline pilocarpine hydrochloride is considered a cholinergic muscarinic agonist that activates muscarinic sensors located in smooth muscles such as the muscles of the iris network and ciliary muscle.Vuity reduces the iris circle muscles and narrows the pupil to improve vision at short distances, but the pupillary response is only slightly protective protected. Vuity also reduces the ciliary muscles, placing the eye in a more myopic position.
12. 3 Pharmacokinetics
Systemic effects of pilocarpine have been observed in 22 presbyopic members who received 1 drop vity of all oko once a day in the direction of 30 days. mean CMAX and AUC0-T, SS on day 30 were 1, 95 ng/ml, 4, 14 ng-H/ml, respectively. mean statement on day 30 was 0, 3 hours after dosing method, 0, 2 to 0, 5 hours after dosing method. mean CMAX and AUC0-T, SS on day 30 were 1, 95 ng/ml, 4, 14 ng-H/ml, respectively.
13 Pre-dose toxicology
13. 1 Carcinogenesis, mutagenesis, and fertility issues
Pilocarpine did not induce tumors in mice for each dose test (up to 30 mg/kg/day, approximately 160 MRHOD). In rats, oral administration of 18 mg/kg/day (approximately 200 MRHOD) produced a statistically significant increase in the incidence of benign follicular cell tumors in both male and female rats and statistically significant adenomas in female rats.
Pilocarpine appeared to be unable to cause genotoxicity in a series of studies including: 1) bacterial testing of circulating geneu (Salmonella and E. coli); 2) live observations on chromosomal aberrations in the Chinese portion of hamsteristics; 3) patients with chromosomal aberrations (micronucleus test) in mice; 4) Early DNA damage test in the early growth of rat hepatocytes (non-centralized DNA synthesis).
Fertility loss.
Oral administration of pilocarpine in male and female rats at a dose of 18 mg/kg/day (200 daily doses recommended for 200 individuals) results in decreased fertility, decreased spermatogenesis, and abnormal sperm morphology indications. It is not known whether the reduced fertility is due to to effects male, female, or both. In dogs, exposure to pilocarpine resulted in a dose of 3 mg/kg/day in the direction of 6 months with a decrease in symptoms of spermatogenesis (approximately 110 more than the recommended daily dose in humans).
14 Clinical Study.
The efficacy of vigor for the treatment of presbyopia was demonstrated in 2 Phase 3, randomized, double-masked, vehicle – 30 days, namely Gemini 1 (NCT03804268) and Gemini 2 (NCT03857542). In the pooled study, 750 members between the ages of 40 and 55 years were randomized in two surveys with presbyopia (from 375 to Vuity Group) and members received instructions one drop from vuity or vehicle, administered once daily in both eyes.
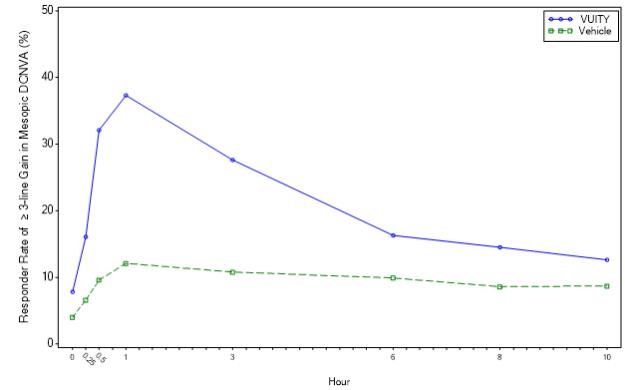
In both studies, a proportion of members in three or more divisions, were given the most contrast-rich, binocular distance corrected near care sharpness (DCNVA). Refractive correction was statistically significantly higher in the vuity group compared to the 30-day, 3-hour vehicle group (see Table 1).
| Gemini 1 | Gemini 2 | |||||
| vituict n = 163 | Vehicle n = 160 | P Value | Vituity n = 212 | Vehicle n = 215 | P Value | |
| Percentage of members who received 3 or more copies in the Mesopic DCNVA will lose no more than 1 copy (5 letters) of the CDVA on day 30 | 31% (5 letters) | 8% | p | 26% (5 letters) | 11% (of members who received more than one copy of CDVA) | p |
Figures 1 and 2 show the sacrifices of members who reached 3 or more copies in the mesopic DCNVA on day 30.
Figure 1: Tolerance of members who improved more than 3 rows in mesopic highest contrast binocular dcnva at day 30 in Gemini 1 (intent to treat population)
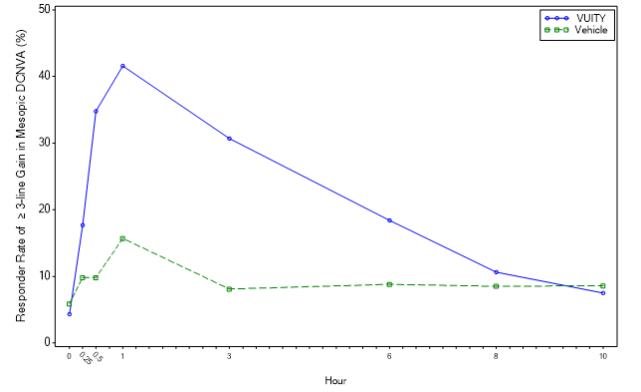
Figure 2: Tolerance of participants who achieved an improvement of 3 or more rows on the mesopic highest contrast binocular dcnva on day 30 of Gemini 2 (intent to treat population)
Delivery/Storage and Treatment Methods
Vitality is provided in the form of sterile eye solution in dull cellophane with low-density (LDPE) ophthalmic dispensing cell flacon and hand paper, light green high-impact polystyrene in the following manner
| 2, 5 ml filled bottles 5 ml (box with 1 bottle) | NDC 0074-7098-01 |
| 2, 5 ml fill 5 ml bottles (box with 3 bottles) | NDC 0074-7098-03 |
| 2, 5 ml bottle filled with 5 ml (box with 2 bottles) | NDC 0074-7098-04 |
Store at 15°C to 25°C (59°F to 77°F). After opening, Vuity can be applied to bottle expiration date.
17 Information on Patient Guidance
Critical features during nighttime operation may cause temporary dim or black view. Caution patient to drive in the dark and with significant exposure in poor light. [See Warnings and Precautions (5. 1)].
Short-term accommodation problems may occur. when changing focus between near and distant objects. Advise patient not to drive or operate machinery if vision is not clear (e.g., blurred vision). [See Warnings and Precautions (5. 1)].
If sudden flashing lights, floaters, or loss of face occur, advise patient to gather medical advice to call for medical assistance immediately. [See Warnings and Precautions (5. 2)].
Before administering Vitualt, contact lenses with contact lenses should be removed. Wait again with contact lenses after 10 minutes of administration. [See Warnings and Precautions (5. 4)].
Prevent product contamination by not touching the airplane drop tip as this may contaminate the contents. [See Warnings and Precautions (5. 5)].
Simultaneous ocular therapy requires that medications be at least 5 minutes apart when multiple timely ophthalmic medications are used.
Distributed by Alleran, Abbvie; prepared by Abbvie Inc. North Chicago, IL 60064 USA
© 2022 Abbvie. All rights reserved. vuity and its design are considered trademarks of Allergan Sales, LLC, an Abbvie company.
Basic Sidewalk Signs
(pilocarpine hydrochloride ophthalmic solution) 1. 25
2. 1 5 ml bottle
For timely use in the eye area
Abbey Company

Basic Sidewalk Signs
(pilocarpine hydrochloride ophthalmic solution) 1. 25
2. 3 5 ml bottles in a packet
For timely use in the eye area
Abbey Company

Basic Sidewalk Signs
(pilocarpine hydrochloride ophthalmic solution) 1. 25
For timely use in the eye area
Abbey Company

Basic Sidewalk Signs
Professional sample not for sale
(pilocarpine hydrochloride ophthalmic solution) 1. 25
For timely use in the eye area
Abbey Company

| Product Information | |||
| Product Type | Human Prescription Drug Label | Substance Code (Source) | NDC: 0074-7098 |
| Dosing Method | Ophthalmology | DEA Schedule | |
| Active ingredient/ Active ingredient | ||
| Ingredient Name | Strength Basis | Courage |
| Pilocarpine hydrochloride (pilocarpine) | Pilocarpine hydrochloride | 12.5 mg in 1 ml |
| Inactive ingredient | |
| Ingredient Name | Courage |
| Boric acid | |
| Trisodium citrate dihydrate | |
| Sodium chloride | |
| Water | |
| Hydrochloric acid | |
| Sodium hydroxide | |
| Benzalkonium chloride | |
| Packaging | |||
| # | Product Code | Package Description | |
| 1 | NDC: 0074-7098-01 | 1 bottle per box | |
| 1 | NDC: 0074-7098-02 | 2.5ml per bottle | |
| 2 | NDC: 0074-7098-03 | 3 bottles per box | |
| 2 | NDC: 0074-7098-02 | 2.5ml per bottle | |
| 3 | NDC: 0074-7098-04 | 1 bottle per box | |
| 3 | NDC: 0074-7098-02 | 2.5ml per bottle | |
| 4 | NDC: 0074-7098-05 | 1 bottle per box | |
| 4 | 1.5 ml per bottle | ||
| Marketing Information | |||
| Marketing Category | Order number or monograph citation | Marketing Start Date | Marketing End Date |
| NDA | NDA214028 | 2021. 10. 28 | |
| Labeler – Abbey Corporation (078458370) |
AbbVie Inc.
Read more about VIUTY (pilocarpine eye drops)
- Observe the interaction
- Pricing and Coupons
- Recent FDA Warnings (1)
- Side effects
- Dosage Information
- Pregnancy
- History of FDA Encouragement of
- Breastfeeding
- Spanish
Resources for Patients
Tools for Professionals
- Prescribing Information
- Pilocarpine Eye Drops (FDA)
Side Effects of Vuity
Medical tests evaluated by drug. Com. Last updated October 12, 2022.
NOTE: There are updates to this document. effect Ophthalmologic information on pilocarpine. Some of the dosages listed on this page may be unfamiliar to some brands
Use of pilocarpine eye drops: ophthalmic solution.
Serious side effects of Vuity
In addition to what is needed, effects pilocarpine ophthalmic (the active ingredient in Vitis) can cause a number of undesirable side effects. effects Not all of these side effects, though, effects occur, medical support may be required.
Contact your physician immediately if any of the following disorders effects occur ophthalmologically during the use of pilocarpine.
More frequent
- Sweating
- Muscle vibration
- Nausea, vomiting, or diarrhea
- Redness of the white part of the eye or the inside of the eyelid
- Shortness of breath
- Water in the mouth
Less common
- Blurred vision
- Irritation or pain in the eyes
- Eye tearing
Frequency unknown
- Junction
- Fast heartbeat
- High temperature
- Hives, itching, skin rash
- Irritation
- Joint pain, stiffness or swelling
- Skin redness
- See flashes or sparks
- See spots floating in front of the eyes or veils or curtains appearing in parts of the landscape
- Swelling of eyelids, face, lips, arms, or legs.
- Shortness of breath
Other side effects of Vuity
Some side effects Ophthalmie Ophthalmie can occur, but usually there is no medical help. These silks effects may decrease during healing, for example, when your body adapts to medications. Additionally, caregivers can talk about techniques to prevent or reduce some of these side effects. effects .
Talk to your care provider as one of the relevant parties effects if you have any questions about it, whether it persists or annoys you:
More frequent
- Reduced night vision
- Headaches
For health care professionals
Pilocarpine Ophthalmic: applied to composite powders, ophthalmic gels, ophthalmic inserts, ocular solutions
Eyeglasses
Often (1% to 10%): adaptive changes, blurred vision, eye discomfort, looking not good, eye pain
Frequent not mentioned: ciliary spasm, pruritus, smart/ burning, conjunctival congestion with sensitization of members, temporary myopia, lens configuration for acquired use, poor light vision, exaggerated pupil block, glass moisture blood].
Frequent
Most common side effects. effects Were violations of adaptive configurations, blurred vision, dissatisfaction with eyes, vision and eye pain. [See also]
Cardiovascular
Frequencies not mentioned: blood pressure configuration, heart rhythm [see also].
Dermatology
Frequency not mentioned: sweating, excessive saliva, left [Ref].
Nervous system
Frequent (1% to 10%): headache, hollow forehead
Frequency not mentioned: tremor [Ref].
Respiratory system
Frequency not mentioned: bronchospasm, edema non-neig [ref].
Gastrointestinal
Frequency not mentioned: nausea, nausea, diarrhea [ref].
Read more about VIUTY (pilocarpine eye drops)
- Observe the interaction
- Pricing and Coupons
- Recent FDA Warnings (1)
- Dosage Information
- Pregnancy
- History of FDA Encouragement of
- Breastfeeding
- Spanish
Resources for Patients
Other Brands
Tools for Professionals
Recommendations
1. “Product Information. Pilopine-HS (Pilocarpine Ophthalmic). “Alcon Laboratories Inc.
2. “Product Data Summary for the United Kingdom” by Cerner Multum, Inc. o 0
Cerner Multum, Inc. “Product Information for Australia”. o 0
More Information
Always consult your health care provider to ensure that the information displayed on this page is used for your own incident.
Some side effects I cannot report this information. You may report this information to the FDA.
Vuity Adverse Reaction Center
Vuity (Pilocarpine Hydrochloride Chloride Solution) gives a cholinergic muscarinic sensor agonist that has been shown to cure mature presbyopia.
What are the side effects of Vitualt?
Side effects of Vuity include:
- Headache and.
- Redness of the eyes.
Vitualt has the ability to cause smooth sides effects including:
- hive,
- Breathing difficulties,
- Swelling of the face, lips, tongue, and throat,
- blurred vision,
- eye pain,
- Visual disturbances,
- Eye irritation and
- Excessive tearing or watering of the eyes
Seek medical assistance immediately in case any of the above signs are present.
Zoek Onmiddellijk Medische Hulp of Bel 911 Geval Van Een Dreigend Medisch Noodle effects :
- ernstige oogsymptomen, Zoals Plotting verlies van gezichtsvermogen, wazig zien, tunnelvisie, oogpijn of zwelling, of het zien van halo’s rond lichten ;
- Ernstige Hartsymptomen, Zoals een Plotselinge, Bonzende Hartslag onregelmatige, Fladderen op de Borst, Kortademigheid en en duizeligheid, richt in het hoofd of flauvallen;.
- Ernstige Hoofdpijn, Verwarde, Onduidelijke Spraak, Moeite Met Lopen, Verlies VanCoördinatie, Eerder Stijve Spieren, Hoogste Koorts, Overvloedig Zweten of Trillen.
dit document bevat niet alle mogelijke bijwerkingen effects en er kunnen nog andere bijwerkingen optreden. Raadpleg je Arts voor aanvullende Informatie over Bijwerkingen. effects .
Voor Vitaliteit dosage
The dose of Vuity is one drop an okogeënt の day あたりの is used once。 If more than 1 timely ophthalmic product is used、the products must be applied for at least 5 minutes。
PRESBYOPIA IN CHILDREN
Aging does not occur in the pediatric population.
What Medications、Drugs or Suppleens Have Interaction With Vuithy?
Vitality May Have an Interaction は、Other Medicines に会いました。
Tell YOUR Physician About All Medicines and Explants You Use。
vitiol during pregnancy and lactation
TELL YOUR OWN ARTS IF YOU ARE Pregnant OR PLANING TO BE Pregnant BEFORE TAKING Vuity; it は unclear what effect this may have on the fetus です。ヘットは、デ・モーダーメルクの Vuity Passes の unclear です。Consult YOUR Physician before breastfeeding。
Additional Information
Our Vuity (Pilocarpine Hydrochloride Chlorine Solution) 1, 25% District Use in Ophthalmology. The Healing Center for Side Effects provides an extensive overview of available information on possible side effects of the product. effects Sego in the form of a drug.
This is not a complete list of side effects effects and others can be acted upon. For medical advice on side effects, call your own physician. effects You can report side effects. You can report side effects effects at the FDA at 1-800-FDA-1088.

Slideshow.
Vuity Professional Information
Patient Information
Riding in the Dark
Vuity may cause temporary blurred or cloudy vision. Advise patient to use common sense when driving in the dark and when performing unsafe activities in poor light. [See Warnings and Precautions].
Regulatory spasms
Changing focus between near and far objects can cause short-term problems. Advise patient not to drive or operate machinery if vision is blurred (e.g., blurred vision). [see Warnings and Precautions].
When to seek medical advice?
Advise the patient to seek medical assistance immediately with sudden special symptoms, blurred vision, or loss of vision. [see Warnings and Precautions].
Contact Lenses
Contact lenses should be removed before Vity is injected. Wait 10 minutes after re-dosing with contact lens. [see Warnings and Precautions].
How to avoid product contamination
Do not touch the tip of the druppelaar with a flat surface. This can lead to contamination of the contents. [See Warnings and Precautions.]
Simultaneous Ophthalmic Treatment in the District
When using more than one ophthalmic product in a district use should be spread out with at least a 5 minute break.

Listen.
©Vuity Patizen information provided by Cerner Multum, Inc. and Vuity Claight information provided by First DataBank, Inc. Used under license and proper copyright.
Wellness Solutions from our sponsors
- My penis is bent over in an erection.
- Can I Get CAD?
- Curved Finger Treatment
- Treat HR+, HER2-MBC
- Fed up with Lou?
- Living with Cancer
Tools for quick identification of pills and routine identification of pills
Interaction tools for medical drugs. Testing for possible interactions with medical medications.
24 hour pharmacy finder for pharmacies






