Many readers are interested in the appropriate topic triamcinolone acetonide toothpaste USP, 0, 1%. I am happy to report that the manufacturer has already done a study of current research on your fascinating subject. We will give you a wide range of answers based on the latest medical reports, advanced research papers, and sample survey information. Keep repeating to find out more.
Each gram triamcinolone acetonide toothpaste USP, 0, 1% has 1 mg triamcinolone acetonide in a dental paste Contains gelatin, pectin, room fragrance, vanilla fragrance, sodium carboxymethyl cellulose, plasticized hydrocarbon gel, polyethylene, mineral oleagel.
Triamcinolone Toothpaste
Triamcinolone (Trye Am Sin Oh Lone) treats and illuminates the discomfort caused by mouth ulcers. It works by reducing inflammation in the mouth. It belongs to a group of drugs called neighborhood steroids.
This medication can be used for other purposes as well. If you have any questions, ask your own health care provider or pharmacist.
Generic Markna(a)m(s): olarone kenalog
What should I tell my care team before I take this medication?
They need to know if you have any of these criteria
- Functional infection
- Stomach or intestinal
- Abnormal or allergic reactions. to triamcinolone Corticosteroids, other medications, foods, dyes or preservatives
- Trying to conceive
- Breastfeeding.
How should I use this medicine?
This drink is used for painful spots in the mouth. Follow the directions on the recipe label. Directions for use the paste After meals and before bed. Use a cotton swab on a small amount of paste on the area to be treated to form a smooth layer. Do not rub. the paste spread or rub in this area as it will become crumbly and sandy. Do not use more often than indicated.
Consult your care team regarding the use of this medication in children. Special precautions may be required.
PERSIDIA: If you suspect you are taking a large dose of this medication, contact the Antigif Center or the emergency department.
NOTE: This medication is for you and you alone. Do not share this medication with others.
What happens if I miss a dose?
If you miss a dose, use it as soon as possible. If it is time for the proper dose, use only that dose. Do not use double or additional doses.
What are the potential interactions with this drug?
No interactions are anticipated.
This list does not cover all possible interactions. Provide the caregiver with a list of all medications, herbs, non-recipe drugs, or supplements that you use. Also tell them if you smoke, drink alcohol, or use illegal drugs. Some things may come in contact with your medications.
What should I pay attention to when using this medication?
Access your care team for ongoing management of your progress. Tell your care team if your symptoms do not improve or begin to worsen. Do not use another medication later without discussing it with your care team. This medication may make some conditions worse.
What side effects can I notice about the use of this medication?
Side effects that should be reported to your care team as soon as possible:
- allergic reactions – skin rash, itching, hives, facial swelling, sponges, tongue, or throat
- Mouth sores, discomfort, or pain in the mouth at the site of application
Side effects that usually do not require medical support (report them to your care team if they persist or are annoying):
- Irritation at the application Web site
This list may not cover all possible side effects. Ask your doctor about side effects; you can report side effects to the FDA at 1-800-FDA-1088.
Where should I keep my medicine?
Save in places that are difficult for children to reach.
Store at room temperature between 15 and 30 degrees C (59 and 86 degrees F). Do not freeze. Protect from light. Discard each unused drink after the expiration date.
NOTE: This journal is to be considered a summary. Not all possible information may have been given. If you have questions about this medication, consult your own physician, pharmacist, or care provider.
Description.
Triamcinolone Acetonide Toothpaste USP, 0, 1% has corticosteroids triamcinolone acetonide with an adhesive that is ideal for use in the tissues of the mouth. Triamcinolone. acetonide Chemically called 9-fluoro-11β, 16α, 17, 21-tetrahydroxyplagna-1, 4-Dies 3, 20-dione cyclical 16, 17-acetal s acetone Field structural formula of triamcinolone acetonide is as follows:
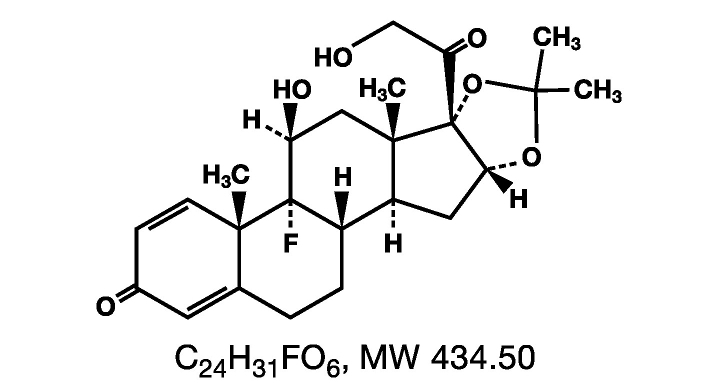
Each gram triamcinolone acetonide toothpaste USP, 0, 1% has 1 mg triamcinolone acetonide in a dental paste Contains gelatin, pectin, room fragrance, vanilla fragrance, sodium carboxymethyl cellulose, plasticized hydrocarbon gel, polyethylene, mineral oleagel.
Clinical Pharmacology
Like other live corticosteroids, triamcinolone acetonide It possesses anti-inflammatory, anti-uric oxidative, and vasoexciting properties. The device of the anti-inflammatory capacity of topical steroids is usually unknown. At the very least, corticosteroids are to function in the induction of phospholipase A 2 inhibitory proteins called lipocortins. These proteins are assumed to regulate the biosynthesis of potent inflammatory media such as prostaglandins and leukotriethenes by releasing the joint precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2 .
PharmacokineticsThe degree of absorption by the oral mucosa is determined for a variety of reasons, including the carrier, the uniformity of the mucosal barrier, the duration of treatment, and the presence of inflammation and/or other diseases. Subsequently, the absorption by the mucosal membrane, the location of the corticosteroid is comparable to the structure of systemically administered corticosteroids. Corticosteroids are bound to plasma proteins to varying degrees. Corticosteroids are metabolized primarily by the liver and excreted by the kidneys. Some corticosteroids and their metabolites are still excreted in bile.
Indications and Uses
Triamcinolone acetonide toothpaste USP, 0, 1% is indicated for the additional healing and temporary illumination of inflammatory lesions of the oral cavity caused by trauma and the drawing associated with ulcerative inflammatory lesions.
Contraindications.
Triamcinolone acetonide toothpaste USP, 0, 1% is contraindicated in patients with hypersensitivity to one of the ingredients of the drug. It is also contraindicated in the presence of fungal, viral, or bacterial infections of the mouth or throat.
Precautions
GENERAL INFORMATION: Triamcinolone Acetonide Toothpaste USP, 0, 1% may cause topical adverse reactions. If dissatisfaction occurs, triamcinolone acetonide – Toothpaste USP, 0, 1% footsteps can be terminated and still establish coherent therapy. Allergic hypersensitivity to corticosteroid contact is usually diagnosed by investigating whether there is no healing instead of observing clinical deterioration, as with most corticosteroid-free neighborhood products. This inadvertence must be demonstrated by appropriate diagnostic patch testing.
If an accompanying mucosal infection is present or has developed, an appropriate antifungal or fungicide should be used. If an appropriate response does not appear immediately, the introduction of triamcinolone acetonide dental paste USP should be stopped 0, 1% until the infection is adequately controlled.
If significant regeneration or recovery of oral tissue has not occurred in the 7-day direction, adjunctive studies on the etiology of oral loss are recommended.
Reversible suppression of the hypothalamic-adrenal axis (HPA), symptoms of Cushing’s syndrome, hyperglycemia, glucosuria, and other less favorable effects due to systemic absorption of topical corticosteroids have made parenterally produced sterionic preparations popular. Thus, it is possible to be present intentionally from time to time to evaluate patients on long-term therapy with corticosteroid support, including corticosteroids dental pastes Informatie Voor dePatiënt: PatiëntenDie RegionaleCorticosteroïderGebruiken, Moeten Goed WoldenGeïnformeerdEnBegeleid:
dit geneesmiddel moet worden toegediend volgens de destersies van de Arts.
Field this is Allen Boa Oraal Gebruik: het is niet gespecialiseerd voor oogheelkundig in dermatologisch gebruik. or dentist PatiëntenMoetworden geadviseerd dit geneesmiddel niet gebruiken voor een andere aandoening dan waarvoor het is voorgeschreven.
PatiëntenMoetenAlle symptomatic Van Bijwerkingen Melden.
Net Als Bij anderecorticosteroïdermoetde Behandeling worden gestaakt wanneer contleol is belake. neem Contact op Met De Arts Als er na 2 Maanden Geen Verbetering optreedt.
Laboratorium control. Urine vrije cortisoltest en acth-stimulatest kunnen nuttig zijn bij het beoordelen van hpa-as onderdrukking. or dentist .
Kansinogese, madagenese, nietenleving vruchtbaarheidsStudies
For the potential to induce carcinogenesis、non-compliance with fertility のマタゲネーゼ triamcinolone acetonide Pregnancy Category C:テラトゲンエフェクテン。トリアムシノロン
it は been shown to cause teratogenic effects in various animal species です。 In Mice and Rabbits、. acetonide Accordingly, doses of approximately 120 µg/kg/day and 24 µg/kg/day caused overestimation of the split palate (approximately 12 and 10 more than normal daily doses of triamcinolone acetonide tooth pasta USP, based on body surface estimates of 0 and 1% respectively after standardized comparison (formal data). With monkeys, triamcinolone acetonide Induces cranioskeletal malformations at the lowest dose studied (500 µg/kg/day). This was about 200 times the normal daily dose of triamcinolone acetonide toothpaste USP, 0, 1%, when comparing data based on in vitro estimates. No adequate and properly controlled studies were conducted on pregnant women. However, in a retrospective study of birth defects born to mothers born to mothers who used the same class of substances as triamcinolone acetonide toothpaste USP, 0, 1% (corticosteroids) showed an approximately threefold increase in the incidence of split mouth. Triamcinolone acetonide toothpaste USP, 0, 1% can only be used during pregnancy if the possible risk to the fetus may justify it. triamcinolone acetonide It is not known whether breastfeeding mothers can produce detectable amounts in breast milk, leading to systemic absorption that would require oral use of corticosteroids. It is prudent to use corticosteroids with caution.
Prescribed to lactating women. dental pastes The safety and efficacy of triamcinolone acetonide toothpaste in children should be used in children. 0 or 1% in children is unknown. Pediatric patients have a higher sensitivity to topical corticosteroid suppression of the HPA axis and Cushing’s syndrome than adult patients because of their higher weight-to-weight skin surface ratio. Enter corticosteroids.
In the limiting footprint, children follow the lowest dose compatible with an effective treatment regime. Acquired corticosteroid therapy may affect the child’s rise and maturation. dental pastes Clinical studies on triamcinolone acetonide toothpaste USP, 0, 1% do not include enough described subjects over 65 years of age to determine if responses differ from younger subjects. Other reports of clinical studies found no differences in response between older and younger patients.
Side Effects.
When corticosteroids are used, neighborhood correct side effects may occur.
Not present before treatment: burning, burning, itching, pruritus, discontent, dryness, blistering, or peeling, peritonitis, allergic contact dermatitis, oral mucosal infiltration, secondary infection, oral mucosal atrophy. dental pastes See also Precautions for possible systemic absorption effects.
Dosing and Administration
Press a small depet (about 1/4 inch) into the lesion until a nice film is created. A larger dose may be needed to cover some lesions. For good results, bring in just enough to cover the lesion with a narrow film. Do not rub. Trying to spread this preparation can lead to a detailed, gritty sensation and will cause it to disintegrate. After use, however, a smooth, itchy film will be created.
Using the preparation during sleep should allow the steroid to come in contact with the loss in the direction of the night. Depending on the severity of symptoms, it may be necessary to use the preparation two to three times a day, preferably after eating; if significant recovery or regeneration has not occurred after seven days, a subsequent examination is recommended.
How supplied.
1% tube of triamcinolone acetonide toothpaste USP, 0, 5 g.
Store hermetically sealed closed; store at 20°-25°C (68°-77°F) excursion and employed at 15°-30°C (59°-86°F) [see USP controlled room temperature]. of dental paste NDC 21695-641-05
Dispersion: Rising Pharmaceuticals, Inc. Allendale, NJ 07401
Manufactured by Lyne Laboratories, Inc. Brockton, Monday 02301
Distributor: Rebel Corporation.
Thousand Oaks, CA 91320
Main Pavement
(triamcinolone pasta)
Drug Medical Testing. com. last updated December 1, 2022.
On this page.
Description
- Clinical Pharmacology
- Indications and Uses
- Contraindications
- Precautions
- Information on patient guidance
- Adverse Reactions
- Medication and Administration
- Delivery/Storage and Handling
- Triamcinolone Toothpaste Description
Triamcinolone acetonide toothpaste USP, 0, 1%, corticosteroid available
with the best adhesive for use in oral tissues. Triamcinolone. triamcinolone acetonide with an adhesive that is ideal for use in the tissues of the mouth. Triamcinolone. acetonide Field structural formula acetone Field structural formula of triamcinolone acetonide is as follows:
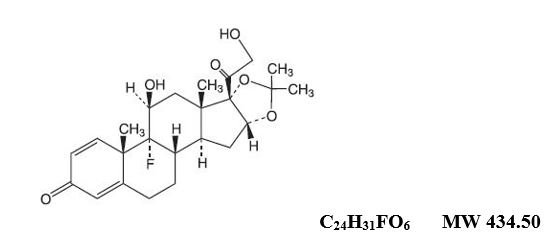
Each gram of triamcinolone acetonide dental paste contains 1 mg triamcinolone acetonide in an emollient dental paste Triamcinolone pasta – clinical pharmacology
Like other live corticosteroids,
Like other live corticosteroids, triamcinolone acetonide It possesses anti-inflammatory, anti-uric oxidative, and vasoexciting properties. The device of the anti-inflammatory capacity of topical steroids is usually unknown. At the very least, corticosteroids are to function in the induction of phospholipase A 2 inhibitory proteins called lipocortins. These proteins are assumed to regulate the biosynthesis of potent inflammatory media such as prostaglandins and leukotriethenes by releasing the joint precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2 .
The extent of absorption by the oral mucosa is determined by a variety of reasons, including the carrier, the uniformity of the mucosal barrier, the duration of treatment, and the presence of inflammation and/or other diseases. The location of corticosteroids subsequently absorbed by the mucosal membrane is comparable to the structure of systematically administered corticosteroids. Corticosteroids are bound to plasma proteins to varying degrees. Corticosteroids are metabolized primarily by the liver and differentiated by the kidneys. Some corticosteroids and their metabolites are still excreted in bile.
Triamcinolone Paste Indications and Dosing
Triamcinolone acetonide toothpaste USP, 0, 1% is indicated for additional healing and temporary illumination of inflammatory lesions of the oral cavity caused by trauma and the drawing associated with inflammatory lesions of ulcerative lesions.
Triamcinolone acetonide toothpaste USP, 0, 1% is indicated for the additional healing and temporary illumination of inflammatory lesions of the oral cavity caused by trauma and the drawing associated with ulcerative inflammatory lesions.
Precautions
Triamcinolone acetonide dental paste Precautions
Information on patient guidance
May cause adverse reactions in the region. In case of dissatisfaction,
Triamcinolone acetonide dental paste use should be discontinued and appropriate treatment instituted. Allergic hypersensitivity to corticosteroid contact is usually diagnosed by studies on lack of healing rather than by finding clinical deterioration, as with most corticosteroid-free topic products. This inadvertence should be demonstrated by appropriate diagnostic patch testing. triamcinolone acetonide dental paste If a mucosal infection is present or develops at the same time, appropriate antifungal or fungicidal agents should be used. If the appropriate answer does not appear immediately, an antifungal or fungicide should be used.
The infection should be stopped until it is adequately controlled. of triamcinolone acetonide dental paste If significant regeneration or recovery of mouth tissue has not occurred within 7 days, adjunctive investigation into the etiology of mouth loss is recommended.
Systemic absorption of topical corticosteroids is popular in pro-dasedawional praxis, including reversible suppression of hypothalamus-viennialis (HPA), Cushing’s syndrome symptoms, hyperglycemia, and glucosuria. Thus, with the support of corticosteroids, it is possible to evaluate patients from time to time on occasional long-term therapy.
Reversible suppression of the hypothalamic-adrenal axis (HPA), symptoms of Cushing’s syndrome, hyperglycemia, glucosuria, and other less favorable effects due to systemic absorption of topical corticosteroids have made parenterally produced sterionic preparations popular. Thus, it is possible to be present intentionally from time to time to evaluate patients on long-term therapy with corticosteroid support, including corticosteroids dental pastes Patient Information
Patients using topical corticosteroids should receive the correct information and package leaflet.
This drug must be used according to the doctor’s instructions
- Field this is Allen Boa Oraal Gebruik: het is niet gespecialiseerd voor oogheelkundig in dermatologisch gebruik. or dentist PatiëntenMoetworden geadviseerd dit geneesmiddel niet gebruiken voor een andere aandoening dan waarvoor het is voorgeschreven.
- Patients should report all symptoms of side effects.
- As with other corticosteroids, treatment should be stopped when control is reached; if there is no improvement after 2 months, contact the physician.
- Laboratorium control. Urine vrije cortisoltest en acth-stimulatest kunnen nuttig zijn bij het beoordelen van hpa-as onderdrukking. or dentist .
Urine-free cortisol testing and ACTH stimulation testing are helpful when evaluating HPA axis depression.
Carcinogenesis, mutagenesis, and fertility issues
No animal studies have been conducted to evaluate
Potential to induce carcinogenesis, mutagenesis, or fertility problems. triamcinolone acetonide Has been shown to cause teratogenic effects in different animal species. Using mice and rabbits,
Triamcinolone acetonide Accordingly, doses of approximately 120 µg/kg/day and 24 µg/kg/day caused overestimation of the split palate (approximately 12 and 10 more than normal daily doses of triamcinolone acetonide tooth pasta USP, based on body surface estimates of 0 and 1% respectively after standardized comparison (formal data). With monkeys, triamcinolone acetonide (In comparison, the data were normalized based on body surface estimates, respectively). Monkeys and, of triamcinolone acetonide dental paste caused cranioskeletal fractures at the lowest dose (500 µg/kg/day), which is about 200 higher than the number at normal daily doses in people. triamcinolone acetonide Data normalized based on body surface estimates for comparison. There are no adequate and properly controlled studies in pregnant women. The fewest are retrospective studies of birth defects among children born to mothers who used the same class of drugs of triamcinolone acetonide dental paste (corticosteroids) during pregnancy increased the incidence of schistosomiasis approximately threefold. Triamcinolone. as triamcinolone acetonide dental paste May only be used during pregnancy if the possible risk to the fetus may justify it. acetonide dental paste Latent mothers.
It is not known whether oral use of corticosteroids may lead to systemic absorption sufficient to produce detectable amounts in breast milk. Caution should be exercised when corticosteroids
prescribed for use during breastfeeding. dental pastes Pediatrics
Safety and efficacy
Unknown in pediatric patients. Pediatric patients probably have a larger skin surface relative to body weight, indicating greater susceptibility to topical corticosteroid induction to the HPA axis and Cushing’s syndrome than adult patients. Corticosteroid penetration of triamcinolone acetonide dental paste children should be limited to the minimum number compatible with an effective treatment regime. Acquired treatment with corticosteroids may disrupt the growth and development of the child. dental pastes Older subjects
We did not include the required number of subjects over the age of 65 to determine if they would respond differently than younger subjects. Separate enrolled clinical skills showed no difference in response between geriatric and younger patients.
Clinical studies of triamcinolone acetonide dental paste Side Effects
The following local side effects may be present at any occasion of acting with corticosteroids
Not present before treatment: burning, burning, itching, pruritus, discontent, dryness, blistering, or peeling, peritonitis, allergic contact dermatitis, oral mucosal infiltration, secondary infection, oral mucosal atrophy. dental pastes See also prophylaxis for possible systemic absorption effects.
Pasta Dosage and Triamcinolone Power
Press a small depet (about 1/4 inch) into the lesion until a nice film is created. More may be needed to cover some lesions. For good results, apply only enough to cover the lesion in a narrow layer. Do not rub. Trying to spread this preparation can lead to a detailed, gritty sensation and will result in disintegration. After use, however, a smooth, itchy film will be created.
The preparation should be used during sleep so that steroids can be contacted with nocturnal loss. Depending on the severity of symptoms, the preparation may need to be used two to three times a day, preferably after meals; if significant recovery or regeneration has not occurred after 7 days, a subsequent examination is recommended.
Triamcinolonpasta administration instructions
Triamcinolone acetonide toothpaste USP, 0, 1% is supplied in the form of a beige scent that is not raised
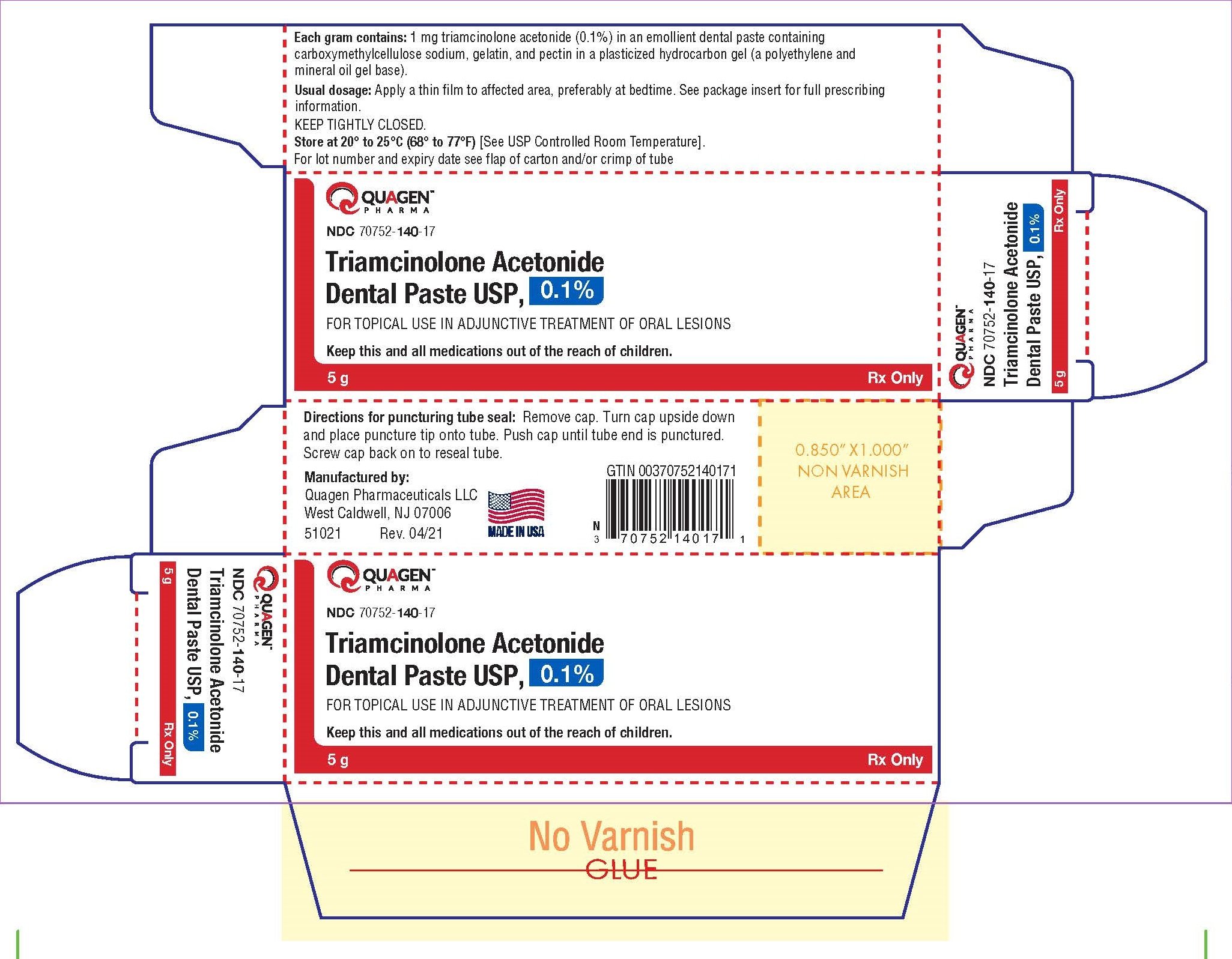
Narrow sandy texture without lumps filled in dural or laminated tubes containing 5 g. paste Laminated tubes (NDC 70752-140-16) and 5 g of dental paste dural tube (NDC 70752-140-17). of dental paste Store closed closed; store at 20°-25°C (68°-77°F) [see USP – see Controlling Room Temperature].
Manufactured by Quagen Pharmaceuticals LLC West Caldwell, NJ 07006
Basic reflective pavilion in 5 g tube with laminate markings
NDC 70752- 14 0-16 Triamcinolone Acetonide Toothpaste USP, only 0, 1% 5g Rx.
5 g laminate label tube NDC 70752- 14 0-16 Triamcinolone Acetonide Toothpaste USP, basic reflective rigor, only 0, 1% 5 g rx.
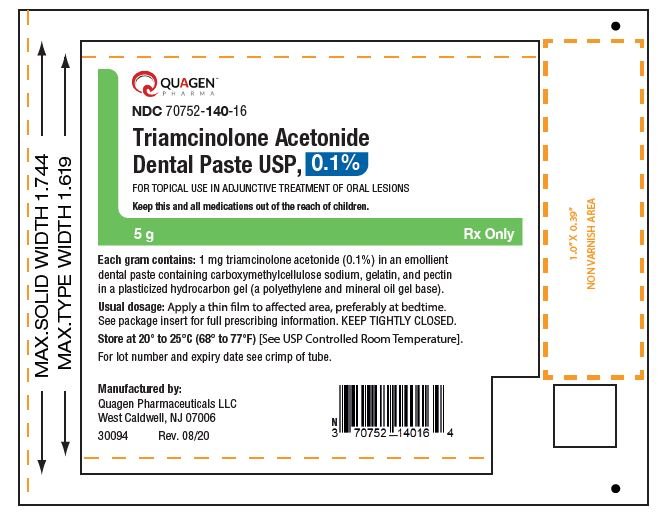
5 g dural plate label basic reflective pavement
NDC 70752- 14 0-17 Triamcinolone Acetonide Toothpaste USP, only 0, 1% 5 g rx
Product Information
| Type | |||
| Human Prescription Drug Label | Dust Code (Source) | NDC: 70752-140 | Administration |
| Dental | DEA Schedule | Active Component/ Active Component | |
| Name Component | ||
| Strength based on | Courage | Triamcinolonacetonide (triamcinolonacetonide) |
| Triamcinolonacetonide | 1 mg in 1 g | Inactive Ingredients |
| Name Component | |
| Strength based on | Triamcinolonacetonide (triamcinolonacetonide) |
| Pectin | |
| Sodium carboxymethylcellulose | |
| Very flexible polyethylene | |
| Mineral oil | |
| Product Properties | |
| Color | |||
| Brown (light beige) | Bill | Shape | |
| Size | Taste | ||
| Code Printing | Includes | ||
| Package | |||
| Product Code | |||
| # | Packaging Description | 1 | |
| NDC: 70752-140-16 | 1 tube per box | 1 | |
| NDC: 70752-140-16 | 2 | ||
| NDC: 70752-140-17 | 1 tube in 1 box | 1 | |
| NDC: 70752-140-17 | 2 | ||
| Marketing Category | |||
| Order number or quoted monograph | Get out of start start | Set end date | And a |
| ANDA214582 | 15. 01. 2023 | LabelGeve r-Quagen Pharmaceuticals LLC (073645339) | |
| Registered Owner – Quagen Pharmaceuticals LLC (073645339) |
| Institution |
| Name | |||
| Address | ID/FEI | Activity | Quagen Pharmaceuticals LLC |
| Production (70752-140), Packaging (70752-140) | 080281331 | Quagen Pharmaceuticals LLC | |
Production (70752-140), Packaging (70752-140)
More about triamcinolone topical
- Compare Alternatives
- Prices & Coupons
- Reviews (97)
- Side Effects
- Dosing Information
- Pregnancy
- Drug Class: Buurtsteroids
- Breastfeeding
- Patient Sources
Tools for Professionals
Prescribing Information
- Triamcinolone (FDA)
- Triamcinolone Lotion (FDA)
- Triamcinolone Ointment (FDA)
- Triamcinolone Spray Can (FDA)
- Triamcinolone Absorbent Base (FDA)
- Structural Formula






