Many readers are interested in Diclofenac Palium-initially, side effects, and almost any other pertinent subject. We are pleased to show you that our manufacturer has already surveyed contemporary research on subjects that are fascinating to you. We will provide you with a wide range of answers based on the latest medical reports, advanced research papers, and sample surveys. Keep reading to find out more.
Stop taking diclofenac And if you notice one of these rare but nonsense side effects, seek medical help immediately: abdominal/stomach pain that does not pass, black/tricky stools, nausea that looks like coffee pigeons, chest/chin/pain in left arm, shortness of breath, unusual sweating, lost, impotence on one side of body, difficulty consulting , unexpected changes in vision.
Information on the purpose of potassium diclofenac.
Drug Medical Testing. com. last updated on December 1, 2022.
On this page.
- Package Warnings
- Description.
- Clinical Pharmacology
- Indications and Uses
- Contraindications
- Warnings
- Precautions
- Information on Patient Guidance
- Medication Interactions
- Adverse Reactions
- Overdose
- Medication and Administration
- Delivery/Storage and Handling
- Medication Guide
WARNING: Risk of Non-Vigorous Cardiovascular and Gastrointestinal Incidents
Cardiovascular Thrombosis Incidents – Steroidal anti-inflammatory drugs (NSAIDs) increase the risk of non-interfering cardiovascular thrombosis, including myocardial infarction and heart attack, which can be fatal. Diclofenac potassium tablets are contraindicated in coronary artery bypass graft (CABG) operations (see Contraindications, Warnings).
Gastrointestinal Bleeding, Ulcers, Perforation NSAIDs cause an increased risk of serious gastrointestinal (GI) side effects, including bleeding, ulcers, and perforations of the stomach or intestinal tract. These side effects have a good chance to run at any time during use and without warning signals. Elderly patients and patients with a history of stomach ulcers or gastric bleeding are at highest risk for GI incidents (see WARNINGS).
Diclofenac Potassium Description
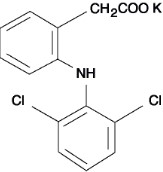
Diclofenac Potassium Tablets, USP are considered a derivative of benzoic acid. Diclofenac Potassium Tablets are available as 50 mg (white to off-white) specific release tablets for oral administration. Diclofenac Potassium, USP is a whitish or broken white or bright yellow crystalline powder, slightly hygroscopic and easily soluble in methanol. It is soluble in alcohol. Low solubility in water; low solubility in acetone. Chemical name 2- [(2, 6-dichlorophenyl)amino]benzoic acid, monopassive salt. Molecular authority is 334, 25. The molecular formula is C 14 H 10 Cl 2 NKO 2 and contains the correct structural formula.
The inactive ingredients in Diclofenac Potassium tablets are lactose monotonate, microcrystalline cellulose, sodium sulfate, colloidal dioxide, magnesium stearate, hypromelisium, hypromellosis, Talk, and titanium dioxide.
Diclofenac – Potassium – Clinical Pharmacology
Diclofenac’s mechanism of action is analgesic, anti-inflammatory, and anti-inflammatory.
As with other NSAIDs, the mechanism of action of diclofenac potassium tablets has not been fully investigated, but includes inhibition of cycloxygenases (COX-1 and COX-2).
Diclofenac is considered a large inhibitor of prostaglandin synthesis in vitro. Diclofenac concentrations achieved during treatment cause in vivo effects. Prostaglandins grow congenital nerves and increase the effect of bradykinin on pain recovery in animal models. Prostaglandins are considered an inflammatory medium. Therefore. diclofenac inhibitors of prostaglandin synthesis, his surgical mechanism may be related to a decrease in prostaglandins in peripheral tissues.
Absorption of diclofenac is 100% after oral administration, as compared to intravenous administration, as measured using urine repair. However, as a result of first-pass metabolism, up to 50% of the absorbed dose is available systemically (see Table 1). For some sober volunteers, measurable plasma values are available within 10 minutes after administration of diclofenac potassium tablets; Piekplasma values are reached in a spectrum of 0.33 to 2 hours, about 1 hour from normal sober volunteers. Ambrosia has no significant effect on the degree of of diclofenac absorption. However, there is usually a delay at the onset of absorption and a decrease in peak plasma values of about 30%.
Table 1. pharmacokinetic characteristics of diclofenac
| Horsepower | Normal healthy adults (ages 20-52) | |
| Average | Variant Ratio (%) | |
| Absolute bioavailability (%) [n = 7] | 55 | 40 |
| t max (hr) [n = 65]. | 1. 0 | 76 |
| Oral clearance (Cl/f; ml/min) [n = 61] | 622 | 21 |
| Renal clearance (% constant product in urine) [n = 7]. | — | |
| Apparent dispersion size (v/f; l/kg) [n = 61]. | 1. 3 | 33 |
| Terminal half-laardetijd (hr) [n = 48]. | 1. 9 | 29 |
The apparent size of potassium diclofenac spread (v/f) formalizes 1, 3 l/kg.
Diclofenac is primarily bound to albumin, more than 99% to human serume protein. Serum protein binding is considered unchanged over the concentration (0, 15-105 µg/ml) reached with the appropriate dose.
Diclofenac diffuses in and out of synovial water. Diffusion in the joint occurs when the plasma value is higher than the synovial water value, then the process is reversed and the synovial water value is higher than the plasma value. Whether distribution to joints plays a role in efficacy is unknown. of diclofenac .
Five diclofenac Metabolites have been identified in human plasma and urine. Metabolites include 4’hydroxy, 5-hydroxy, 3′-hydroxy, 4 ‘, 5-dihydroxy, 3’hydroxy-4 ‘methoxy – diclofenac. Major diclofenac metabolite 4’hydroxy – diclofenac has fairly weak pharmacological potency; the formation of 4’hydroxy – diclofenac is mediated primarily by CYP2C9. Both diclofenac oxidative metabolites then undergo glucuronidation or sulfation with subsequent gallbladder isolation. acylglucuronidation via UGT2B7 and oxidation via CYP2C8. in diclofenac Metabolism. the CYP3A4 is responsible for the formation of the small metabolites 5-hydroxy and 3’-hydroxy-diclofenac. In patients with renal dysfunction, peak concentrations of 4 ‘hydroxy and 5-hydroxy-diclofenac metabolites were about 50% and 4% of the original connection after a one-time oral dose, compared to 27% and 1% in normal healthy subjects.
Diclofenac is eliminated by metabolism and further urinary and gallbladder isolation of the conjugated metabolites of glucuronide and sulfate. Little or no change is diclofenac excreted in the urine. Approximately 65% of the dose is excreted in urine and approximately 35% is excreted unchanged in the bile. diclofenac Plus metabolites. Since renal elimination is not considered an important method of elimination unaltered diclofenac no dosage adjustment is required for patients with impaired to moderate renal function. Unchanged terminal half-life. diclofenac Approximately 2 hours.
Pediatric: the pharmacokinetics of diclofenac potassium pills have not been studied in pediatric patients.
RAS: Pharmacokinetic differences as a result of variety have not been identified.
Hepatic dysfunction: liver metabolism is almost 100% eliminated with diclofenac potassium tablets. Therefore, diclofenac potassium doses should be reduced in patients with liver disease compared to patients with normal liver function.
Renal dysfunction: the pharmacokinetics of diclofenac have been studied in individuals with renal dysfunction. There is no difference in pharmacokinetics of diclofenac identified in studies of patients with renal dysfunction. In patients with renal dysfunction (60-90, 30-60-60, <30 ml/min, n = 6 in each group), AUC and efflux rates were comparable to those of healthy subjects.
Medical Science Promotion
Voriconazole: When given concurrently with voriconazole (CYP2C9, 2C19 and 3A4 enzyme inhibitor), the names C MAX and AUC of diclofenac increased by 114% and 78%, respectively (see PRECAUTIONS; see Drug Interactions).
Aspirin: When NSAIDs were administered with aspirin, protein binding of the NSAID was decreased, but clearance of the free NSAID was not altered. The clinical significance of this interaction is not popular; see Table 2 for clinically significant drug interactions between NSAIDs and aspirin (see Precautions, Medical Interactions).
Diclofenac Potassium Indications and Dosing
Before making a decision about the use of diclofenac potassium tablets, carefully consider the superior properties and risks of diclofenac specific release tablets and other medications. Use the lowest effective dose for the shortest time according to the individual patient’s treatment goals (see Warnings, Gastrointestinal Bleeding, Ulcers, and Perforation).
Diclofenac potassium pills are indicated
- For healing primary dysmenorrhea
- For illumination of non-elevated to moderate pain
- For relief of symptoms and signs of osteoarthritis
- For relief of symptoms and signs of rheumatoid arthritis
Contraindications
Diclofenac potassium tablets are contraindicated in emerging patients:
- Well-known hypersensitivity reactions (e.g., anaphylactic reactions or non-falsified skin reactions) to diclofenac or all possible components of the drug (see WARNINGS, ANAPHYLACTIC REACTIONS, NONFULSIVE SKIN REACTIONS).
- Asthma, ur measles or allergies – history of different reactions after using aspirin or other NSAIDs. Fatal anaphylactic reactions to NSAIDs may be reported in these patients (see WARNINGS, Anaphylactic Reactions, Asthma exacerbations related to sensitivity to aspirin).
- Included in criteria for aortic coronary artery bypass graft surgery (CABG) (see WARNINGS, Cardiovascular Thrombotic Events)
Warnings
Cardiovascular Thrombotic Events
Several COX-2 selective and nonselective NSAID clinical trials of up to 3 years have shown an increased risk of nonfatal cardiovascular (CV) thrombotic events, such as myocardial infarction (MI) and heart attack, and these events are well that they are fatal. Based on the available data, it is unclear whether the risk of cardiovascular thrombotic events can be considered equivalent for all NSAIDs; the conditional increase in the incidence of nonfatal cardiovascular thrombotic events from baseline caused by NSAID use has not been demonstrated for people with common cardiovascular disease or cardiovascular risk factors appears to be the same for those with and without risk factors. However, patients with common cardiovascular disease or risk factors had a higher overall incidence of nonfatal cardiovascular thrombotic events because of the higher initial incidence. Several observational studies have shown that the increased risk of non-fatal cardiovascular thrombotic events began as early as the first few weeks of treatment. The increased risk of cardiovascular thrombotic events was more pronounced at higher doses.
To minimize the possible risk of adverse cardiovascular events in patients receiving NSAIDs, the shortest effective dose should be used for the shortest possible duration. Physicians and patients should be concerned about the development of these events during treatment, even in the absence of previous cardiovascular symptoms. Patients should be informed of the signs of cardiovascular events and the steps to take if a cardiovascular event occurs.
There is no convincing evidence that concurrent aspirin use reduces the increased risk of non-serious cardiovascular thrombotic events associated with NSAID use. Concurrent use of aspirin and NSAIDs, for example as diclofenac Increased risk of non-serious gastrointestinal events (see WARNINGS: Gastrointestinal bleeding, ulceration and perforation).
Status Two large controlled clinical trials of COX-2 selective NSAIDs for pain relief in the first 10-14 days after aortocoronary bypass surgery (ACS) found an increased incidence of myocardial infarction and heart attack NSAIDs are contraindicated in ACS (see Contraindications).
An observational study of post-MI patients in the Danish National Registry showed that patients treated with NSAIDs after MI were at risk for new exposures, central heating-related mortality, and all-cause mortality from the first week of treatment. Similarly, the incidence of death in patients treated with NSAIDs after MI 20 per 100 per year compared to 12 per 100 per year in patients exposed to NSAIDs that were not used. The clear mortality rate declined somewhat after the first year of MI, but the increased comparative risk of death in NSAID users remained at least 4 years of follow-up studies.
Avoid use of diclofenac potassium tablets in patients with recent MI. unless the benefit is expected to outweigh the risk of recurrent central heating thrombotic events. If diclofenac potassium tablets are used in patients with recent MI, check with the patient for symptoms of cardiac ischemia.
Gastrointestinal bleeding, ulceration, and perforation of NSAIDs, diclofenac NSAIDs cause serious gastrointestinal (GI) side effects, including inflammation, bleeding, ulcers, perforation of the GI tract, stomach, small intestine or colon. These serious side effects can occur at any time in patients treated with NSAIDs, with or without warning signals; only every fifth patient who develops a significant side effect in the upper GI during NSAID therapy is considered symptomatic. upper GI ulcer caused by an NSAID, Severe bleeding or perforation occurred in about 1% of patients treated for 3 to 6 months and in about 2 to 4% of patients treated for 1 year. However, even short-term therapy is not without risk.
Risk factors for bleeding, ulcers, and perforation in perforated patients with a history of gastric ulcer and/or gastrointestinal bleeding with NSAID use were compared to patients without these risk factors to increase the risk of gastrointestinal bleeding by more than 10 times. other risk factors for gastrointestinal bleeding in patients treated with NSAIDs points are longer duration of NSAID therapy and concurrent use of oral corticosteroids, aspirin, anticoagulants, or selective serotonin reaffirmation inhibitors (SSRIS). Smoking, alcohol consumption, advanced age, and general poor health. The majority of mail marketing notifications of fatal GI events were made in older or weakened patients. In addition, patients with advanced liver disease and/or coagulopathy are at increased risk for gastrointestinal bleeding.
Strategies to minimize the risk of GI in patients treated with NSAIDs:
- Use the lowest effective dose for the most likely maximum duration.
- Avoid administering more than 1 NSAID at the same time.
- Avoid use in high-risk patients unless it is known that the benefit does not outweigh the increased risk of bleeding. In these patients and in patients with severe gastrointestinal bleeding, alternative therapy to NSAID.
- Pay attention to the draw and symptoms of GI ulcers and bleeding during NSAID therapy.
- If a significant unwanted GI event is anticipated, evaluation and healing should be intensified immediately and diclofenac-potassium tablets should be discontinued until the GI event is substantially ruled out.
- Under the criteria for concurrent use of aspirin at low doses for cardiac prophylaxis, patients should be checked more closely for the presence of indications of gastrointestinal bleeding (see Precautions; Drug Interactions).
In clinical studies of diclofenac with the product, significant increases in aspartate aminotransferase (also called SGOT) (i.e., more than three times the upper limit of [ULN] for [ULN]) were observed. Treatment (alanine aminotransferase [ALT] was not measured in all studies). diclofenac Treatment (alanine aminotransferase [ALT] was not measured in all studies).
In a large open-label controlled study of 3700 patients with oral diclofenac sodium was administered for 2-6 months, patients were first checked after 8 months and again after 24 weeks in 1200 patients. significant increases in ALT and/or AST occurred in about 4% of patients and included clear increases (8 or more ULNs) in about 1% of 3700 patients The public survey showed that the highest borderline increases occurred in about 4% of the patients. In this open study, the highest incidence of borderline (less than 3 times the ULN), small (3 to 8 ULNs), and indicated (>8 ULNs), increases in ALT or AST were increased in patients with a higher incidence alt or ast. diclofenac Compared to other NSAIDs. Increased transaminases were observed more frequently in patients with osteoarthritis than in patients with rheumatoid arthritis.
Almost all significant increases in transaminases were found before patients became symptomatic. Abnormal studies occurred during the first two months of treatment diclofenac 42 out of 51 patients in all studies in which these transaminases increased.
Drug induced hepatotoxicity was reported in the first month of post marketing reports and possibly in the first two months of treatment, but has the opportunity to run at any time during healing. diclofenac . Cases of hepatotoxic reactions such as hepatic necrosis, jaundice, immediate hepatitis with or without jaundice, and hepatic insufficiency have been reported during postmarketing surveillance. Some of these reported cases have resulted in death or liver transplantation.
A European retrospective population-based case-control study enrolled 10 cases. of diclofenac associated with drug-related liver injury with and without current use. of diclofenac It was associated with a 4-fold adjusted odds ratio of statistically significant liver injury. This definitive study was based on a total of 10 cases associated with liver injury. diclofenac Adjusted odds ratios further increased with gender female, dose >150 mg, and duration of use >90 days.
Physicians should initially and occasionally measure transaminase levels in patients on long-term therapy. diclofenac The timing of the first transaminase measurement is unknown. The exact time of the first and subsequent transaminase measurements is unknown. Based on clinical studies and post-marketing experience, transaminases should be monitored for 4 to 8 months after initiation of product therapy. diclofenac However, liver transaminases should not be measured at any time during product treatment. However, liver reactions may occur at any time during product treatment. diclofenac .
The time has come when differences from recognized liver test measurements persist or worsen, when clinical symptoms and/or signs consistent with liver disease develop, or when systemic symptoms (e.g., increased eosinophilia, rash, abdominal pain, diarrhea, black urine) occur. Discontinue prescribing diclofenac potassium tablets immediately.
Inform the patient of any perceived hepatotoxic symptoms and signs (e.g., drowsiness, fatigue, lethargy, diarrhea, pruritus, jaundice, tenderness under the right ribs, signs of influenza). If clinical signs and symptoms consistent with liver disease or systemic symptoms (e.g., eosinophilia, rash, etc.) occur, discontinue diclofenac potassium tablets immediately and examine the patient clinically.
To minimize the risk of unnecessary liver disease in patients taking diclofenac potassium tablets, the shortest effective dose should be used for the shortest possible time. Use diclofenac potassium tablets with caution when used concurrently with drugs known to have hepatotoxic potential (e.g., acetaminophen, medications, antiepileptic drugs).
NSAIDs for hypertension, including diclofenac potassium tablets, may lead to the development of new hypertension or exacerbate existing adverse hypertensive trends. In patients using angiotensin-converting enzyme (ACE) inhibitors, thiazide diuretics, or running diuretics, NSAIDs are not responsive to these therapies (see “Precautions,” “Drug Interactions”).
Check blood pressure (BP) at the start of and during NSAID therapy.
Heart Deficiency and Edema. a meta-analysis of randomized controlled trials with COXIB and classic NSAIDs showed that patients treated with COX-2 selective versus nonselective NSAIDs had approximately twice as many hospitalizations for heart deficiency as those receiving placebo. . Patients. In a study by the Danish National Heart Deficiency Registry, NSAID use increased the risk of MI, heart failure hospitalizations, and death.
In addition, some patients who use NSAIDs experience water retention and edema. use of NSAIDs. of diclofenac may reduce the cardiovascular effects of some therapeutic agents used to treat these conditions (e.g., diuretics, ACE inhibitors, or angiotensin blocking drugs [ARBs]) (see “Precautions,” “Medical Interactions”).
Avoid the use of diclofenac potassium tablets in patients with severe heart deficiency. If diclofenac potassium tablets are used in patients with severe cardiac deficiency, patients should be checked for symptoms of a downward shift in cardiac deficiency.
Nephrotoxicity and hyperkalemia. Kidney. prolonged use of NSAIDs has resulted in necrosis of the renal papillae and other renal injuries.
Nephrotoxicity was also observed in patients in whom renal prostaglandins played a compensatory role in maintaining renal perfusion. In these patients, NSAID use can cause a dose-dependent decrease in prostaglandin formation and renal circulation, which may cause renal compensation. Patients with impaired renal function, dehydration, decreased blood volume, cardiac insufficiency, delivery function, diuretic and ACE inhibitor or BPA use, and the elderly are at increased risk for this reaction. cessation of NSAIDs is usually accompanied by recovery and healing.
There is no information available on the use of diclofenac potassium tablets in patients with advanced renal disease in controlled clinical studies. The renal effects of diclofenac potassium tablets may accelerate the progression of renal dysfunction in patients with pre-existing kidney disease.
Correct renal conditions in dehydrated or hypovolemic patients before starting with diclofenac potassium. Prognosis of renal function in patients with renal or hepatic dysfunction, cardiac deficiency, dehydration, or hemopenia, use of diclofenac potassium tablets (see intermedicinal precautions). Avoid use of Diclofenac Potassium Tablets in patients with advanced kidney disease unless it is assumed that the superior properties do not outweigh the risk of reversing renal function. If diclofenac potassium tablets are used in patients with advanced kidney disease, ensure the patient that renal function is present.
Increased serum levels due to hyperkalemia potassium Even in some patients without renal dysfunction, concentrations masking hyperkalemia have been reported with NSAID induction. In patients with normal renal function, these effects are associated with hypocutaneous cutaneous hyperlordosteronism.
Anaphylactic ReactionsDiclofenac has been associated with anaphylactic reactions in patients with and without known hypersensitivity to diclofenac And in patients with aspirin-sensitive asthma (see Contraindications, Warnings, and Asthma Experimental Therapy Related to Aspirin Sensitivity).
Asthma causation associated with aspirin sensitivity. A subpopulation of asthmatics has aspirin-sensitive asthma, which can lead to sinusitis complicated by nasal polyps. difficult and possibly fatal bronchospasm. and/or Aspirin and other NSAID intolerance. Diclofenac potassium tablets are contraindicated in patients with this form of aspirin sensitivity because cross-reactivity between aspirin and other NSAIDs has been reported in these aspirin-sensitive patients (see Contraindications). If diclofenac potassium tablets are used in patients with pre-existing asthma (without known sensitivity to aspirin), patients are checked for asthma symptoms and changes in symptoms.
Severe skin reactions with NSAIDs, what dose diclofenac It can cause serious side effects such as demodicosis, Stevens-Johnson syndrome (SJS), toxic epidermal necrosis (10), which is fatal. These nonsense actions have the chance to happen without warning. Inform the patient of the signs and symptoms of a non-serious skin reaction and stop taking diclofenac potassium tablets at the first occurrence of a skin rash or other symptoms of hypersensitivity. Diclofenac potassium tablets are contraindicated in patients with a history of severe skin reactions to NSAIDs (see Contraindications).
Medical Drug Reactions Due to Eosinophils and Systemic Phenomena (Dressing)
Medical reactions with eosinophilia and systemic manifestations (connections) have been reported in patients taking NSAIDs such as diclofenac potassium tablets. Some of these incidents were fatal or non-spreading. Fever, skin rash, lymph node tumors, and/or facial edema often occur, but are the least common. Other clinical manifestations are hepatitis, nephritis, blood abnormalities, myocarditis or myositis. Symptoms of connection may resemble acute viral infection. Eosinophilia is often present. Other organ systems may be involved, as this condition is variable in presentation. It is important to note that early signs of hypersensitivity, such as rash and lymphadenopathy, may be present even if the rash is not obvious. If these symptoms or signs are present, stop the diclofenac potassium pills and take the patient away immediately.
Premature Closure of the Fetal Ductus Arteriosus
Avoid the use of NSAIDs in women who are pregnant within 30 months of conception in doses of diclofenac potassium tablets.NSAIDs increase the risk of premature closure of the fetal ductus arteriosus during this gestational period in diclofenac potassium tablets.
oligohydramnios/ neonatal kidney damage
Induction of NSAIDs at diclofenac potassium tablet doses after approximately 20 months of gestation can cause renal insufficiency in the fetus, resulting in amniotic fluid hypersensitivity and possibly renal insufficiency in the newborn. These side effects occur, on average, days to months after the start of treatment, whereas amniotic fluid hypersensitivity is rarely reported 48 hours after the start of NSAIDs. Amniotic fluid deprivation often, but not always, resolves when treatment is discontinued. Long-term worsening of amniotic fluid deprivation may be, for example, limb contractures or delayed maturation of less severe amniotic fluid deprivation. Post-marketing neonatal renal dysfunction may require invasive procedures such as exchange transfusions or dialysis.
If NSAID therapy is required between 20 and 30 months of gestation, diclofenac potassium tablet restriction is used at the lowest effective dose and shortest duration. If diclofenac potassium takes longer than 48 hours to heal, ultrasonic prediction of amniotic fluid should be distinguished. Discontinue diclofenac potassium tablets in the event of amniotic fluid under- or looming symptoms according to medical practice (see Pregnancy Precautions).
Hematologic Toxicity Anemia has occurred in patients receiving NSAIDs. This may be due to occult or hard blood, water retention, or poorly described effects on red blood cell production. If a patient receiving diclofenac potassium tablets has symptoms or signs of anemia, hemoglobin or hematocrit should be monitored.
NSAIDs such as diclofenac potassium tablets may increase the risk of bleeding. Common symptoms include coagulopathy, concomitant use of warfarin and other anticoagulants, anticoagulants (such as aspirin), serotonin reuptake inhibitors (SSRIS), serotonin norepinephrine reuptake inhibitors (SNRIS). Monitor these patients for bleeding symptoms (see Precautions, Drug Interactions).
Precautions
General
Do not expect fast-release tablets of diclofenac potassium to restore corticosteroid effects or treat corticosteroid deficiency. Abrupt discontinuation of corticosteroids may lead to worsening of disease. Patients receiving long-term treatment with corticosteroids should delay the decision to discontinue corticosteroids, and patients should follow the footsteps of closely monitoring symptoms of side effects such as adrenal insufficiency and worsening signs of arthritis.
The pharmacological ability of diclofenac potassium pills in reducing fever and inflammation may reduce the usefulness of these study symptoms in detecting suspected complications of non-infectious painful disorders.
Patient Information
Advise the patient to read the patient sign (medical guide) approved by the FDA belonging to all recipes dispersed. Advise the patient, his/her family or parents about the appropriate information prior to treatment with diclofenac potassium tablets.
Cardiovascular Thrombosis Action advises patients to watch for signs of cardiovascular thrombotic events, such as chest pain, shortness of breath, impotence or voice.
Gastrointestinal bleeding, ulceration, and perforation advises patients to discuss signs of ulceration and bleeding with their care provider, such as ulcer pain, indigestion, melena, and hemolysis. The criteria for concurrent use of aspirin at low doses for cardiac prophylaxis informs patients of the increased risk of symptoms and signs of gastrointestinal bleeding (see Warnings, Gastrointestinal Bleeding, Ulcers, and Perforations).
Hepatotoxicity informs the patient about warning signals and symptoms of hepatotoxicity (drowsiness, fatigue, lethargy, teeth, diarrhea, yellow und, sensitivity to right quadrant and “flu” symptoms). If these occur, instruct the patient to stop diclofenac potassium pills and work on drug therapy (see Warnings, Hepatotoxicity).
Patients with cardiac insufficiency and edema should be alert for signs of congestive heart failure, including shortness of breath, inexplicable weight gain, and edema, and should contact their care provider if these signs occur (see WARNING; Cardiac Inadequacy and Edema).
An anaphylactic reaction alerts the patient to symptoms of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). Instruct the patient to seek immediate emergency help if these symptoms occur (see WARNINGS; see ANAPHYLACTIC REACTIONS).
Severe skin reactions; in which case, the jacket advises the patient to stop diclofenac potassium pills immediately if a skin rash or fever develops and to contact their care provider as soon as possible (see WARNINGS; see SEVERE SKIN REACTIONS).
Female fertility recommends that fertile girls who want to conceive on NSAIDs, including diclofenac potassium tablets, may be associated with reversible delay of ovulation (see precautions; carcinogenesis, mutagenesis, birth defects).
Ask pregnant women to avoid the use of diclofenac potassium tablets and other NSAIDs from 30 months of pregnancy because of the risk of early fetal arterial occlusion, with fetal toxicity paying. Ask if diclofenac potassium tablets need to be given to pregnant women between 20 and 30 months that oligohydramnios should be checked if treatment lasts longer than 48 hours (see Warnings, Fetal Toxicity, Precautions, Pregnancy).
Concurrent use of NSAIDS avoids patients avoiding concurrent diclofenac potassium tablets with other NSAIDs or salicylates (e.g., diflunesate, salsalate). Operation not significantly increased due to increased risk of gastrointestinal toxicity (see Operation not available (inoperable) WARNING; Gastrointestinal bleeding and perforation and drug interactions). (Warning; gastrointestinal bleeding, ulcers and perforation, and drug interactions).Patient convictions that NSAIDs may occur with drugs freely available for the treatment of colds, fevers, or insomnia.
Use of NSAIDs and aspirin in low-dose patients are told not to use aspirin at low doses at the same time as diclofenac potassium tablets until they have spoken with their own caregivers (see interdrug precautions).
The pharmacologic ability of diclofenac potassium tablets in reducing fever and inflammation, and possibly fever, may reduce the ease of use of these laboratory symptoms in detecting infection.
Laboratory predictions should consider support from CBC and chemical profiles for the prognosis of patients treated with NSAIDs, as gastrointestinal bleeding, hepatotoxicity, and kidney damage can occur without warning signals or symptoms (see WARNINGS Bleeding).
Medication Interactions
See Table 2 for clinically important drug interactions diclofenac .
Table 2: Clinically Important Drug Interactions with Diclofenac
- Diclofenac and anticoagulants, such as warfarin, have a synergistic effect on bleeding. The simultaneous use of of diclofenac anticoagulants also increases the risk of major bleeding compared to the use of one of the two medications.
- The release of serotonin by platelets plays an important role in hemostasis. Case-control and cohort pideology studies have shown that concurrent use of NSAIDs with inhibitors of serotonin reinjection may increase the risk of bleeding more than monotherapy with NSAIDs.
- NSAIDs can reduce the antihypertensive effects of angiotensin-converting enzyme (ACE) inhibitors, angiotensin blocking drugs (ARBs) or beta-blockers (including propranolol).
- In elderly patients, concurrent use of an NSAID with an ACE inhibitor or ARB, accompanied by decreased water volume (any amount of diuretic therapy) or decreased renal function, may lead to an increase in negative trends in renal function, possibly including acute renal failure. . These effects are usually reversible.
- The simultaneous use of diclofenac potassium tablets and an ACE inhibitor, BPA or beta-blocker should be used to check blood pressure to ensure that the desired blood pressure is achieved.
- Concurrent use of diclofenac potassium tablets and ACE inhibitors or BPA in elderly patients, patients with decreased water separation, or patients with reduced renal function should be monitored for symptoms of shifts in renal function to the worse side (see WARNING; renal toxicity and hyperkalemia).
- Patients must be adequately hydrated when these substances are used concurrently. Evaluate renal function at the start of concurrent therapy and sometimes thereafter.
NSAIDs with short elimination half-lives (e.g., indomethacin) go with other NSAIDs. NSAIDs with short elimination half-lives (e.g., indomethacin) should be used with other NSAIDs. diclofenac (indomethacin) should be ignored 2 days before and 2 days after the start of pemetrex.
Carcinogenesis, mutagenesis, birth defects.
Carcinogenesis. Long-term studies of carcinogenicity have been conducted in rats. diclofenac Investigations of up to 2 mg/kg/day (based on body surface (BSA) comparisons of the maximum dose (MRHD) of diclofenac potassium tablets in people (MRHD), 200 mg/day) have shown no significant increase in tumor incidence. A 2-year carcinogenicity study in mice diclofenac Doses of sodium of up to 0. 3 mg/kg/day in males (approximately 0. 007 MHRD based on PPCA matching) and 1 mg/kg/day in females (approximately 0. 02 MHRD based on PPT matching) showed no tumorigenic potential.
Diclofenac sodium mutagenicity did not show mutagenicity in in vitro point mutation tests in mammalian test systems (Murien lymphoma) and microbial test systems (yeast, AME). Letale and the survey study chromosomes are overtaken. Studies in mice and nuclear and chromosomal aberrations in Chinese hamsters.
A reduction of fertility diclofenac sodium administered to male and female rats at 4 mg/kg/day (approximately 0, 2 times the MRI based on BSA comparisons) had no effect on birth rates.
Based on mechanism of action, the introduction of prostaglandin-mediated NSAIDs, including diclofenac potassium tablets, may stop or prevent ovarian follicle rupture, which is associated with reversible infertility in some women. Animal studies in place have shown that infiltration of inhibitors of prostaglandin synthesis may not sustain the follicle rupture that is critical for prostaglandin-mediated ovulation. However, a small study of women treated with NSAIDs has shown a reversible delay in ovulation; take out the NSAID face and look at women who have had problems, or have been tested for infertility, with that amount of diclofenac potassium pills.
Pregnancy.
Short-term administration of NSAIDs can lead to premature closure of fetal arteries and fetal kidney damage in that dose of diclofenac potassium tablets, and in some cases to neonatal renal dysfunction. Because of these risks and the limited dosage and duration of diclofenac potassium tablets, diclofenac potassium tablets should be used in approximately 20 and 30 months of pregnancy, and use of diclofenac potassium pills should be avoided in approximately 30 months of pregnancy and the second half of pregnancy (see WARNING; Fetal Toxicity).
Premature Closure of the Fetal Ductus Arteriosus, Introduction of NSAID in the form of diclofenac potassium pills increases the risk of premature closure of the fetal ductus arteriosus at approximately 30 months of gestation or later in pregnancy.
Neonatal induction of NSAIDs in oligohydramnios/about 20 months of gestation or post-pregnancy renal abnormalities has been associated with oligohydramnios and, in some cases, variants of fetal renal dysfunction leading to renal insufficiency in the neonate. There are no adequate and properly controlled studies on diclofenac potassium tablets in pregnant women.
Data from observational studies on the relatively likely embryonic risks of NSAID use in women in the first trimester or gestational period do not provide a definitive answer. Animal reproduction studies have observed virtually no teratogenic symptoms in mice, rats, or rabbits receiving the product. diclofenac (Without considering the presence of maternal and fetal toxicity at these doses, diclofenac potassium tablets are very appropriate for people in the very maximum dose (MRHD) up to about 0, 5, 0, 5, and 1 in the organogenesis stage (MRHD) fact).
Based on animal data, prostaglandins have been shown to play an important role in endometrial vascular permeability, blastocyst implantation, and desmoplasia. In animal studies, the introduction of inhibitors of prostaglandin synthesis, such as inhibitors of prostaglandin synthesis, increased endometrial vascular permeability and the rate of desquamation. as diclofenac This resulted in many losses before and after implantation. Prostaglandins have also been shown to play an important role in fetal kidney development. Animal studies reported no effect on kidney development when prostaglandin synthesis inhibitors were administered at clinically significant doses.
The estimated background risk of nonfatal birth defects and miscarriages in this population is unknown. All pregnancies have a background risk of birth defects, fetal loss, or other adverse outcomes. In the general U.S. population, the estimated background risk varies from 2% to 4% and 15% to 20% from clinically confirmed nonfatal birth defects and miscarriages of pregnancy, respectively.
Fetal/Newborn Adverse Effects
Premature closure of the fetal ductus arteriosus:
Avoid use of NSAIDs in women within the first 30 months of pregnancy and in the second half of pregnancy because NSAIDs, including diclofenac potassium tablets, can cause premature closure of the ductus arteriosus in the fetus (see WARNINGS; see Fetal Toxicity).
Malopecia/Neonatal Renal Dysfunction:
If NSAID is needed after 20 months, limit use to lower doses and for less likely periods of use. If treatment with diclofenac potassium tablets lasts longer than 48 hours, consider possible ultrasound prognosis in cases of low birth weight. In cases of low birth weight, stop diclofenac potassium tablets and continue treatment according to medical practice (see WARNING; Fetal Toxicity). Facts.
Premature closure of the fetal ductus arteriosus:
Available literature suggests that NSAID use during the first 30 months of pregnancy and post-pregnancy may cause premature closure of the fetal ductus arteriosus.
Malopecia/Neonatal Renal Dysfunction:
Published studies and mail marketing reports describe the introduction of Matherna NSAIDs in approximately 20 months or later in pregnancy associated with oligohydramniosis and in some cases fetal renal function leading to neonatal renal failure. These side effects occur on average after days to months of treatment, although oligohydramniosis may be reported 48 hours after initiation of NSAID. Often, but not entirely, the decrease in amniotic fluid was temporary and reversible when the product was stopped.With the exception of oligohydramnio, reports of maternal use of NSAIDs and neonatal renal dysfunction are limited, some of which were irreversible. Some cases of neonatal renal dysfunction requirement for treatment by invasive procedures such as exchange transfusion or dialysis.
Methodological limitations of these post-marketing studies and reports include lack of control groups, limited information on dose, duration, and timing of drug effects, and concurrent introduction of other medications. These limitations make it impossible to produce robust risk assessments of less favorable fetal and neonatal outcomes in the introduction of NSAIDs to mothers. Because the reported data on protection in neonatal outcomes relate primarily to the time of onset, generalization of specific reported risks to infants exposed to NSAIDs through maternal administration is considered uncertain.
Animal reproduction and development studies indicate that diclofenac Sodium hospitalization during organogenesis did not cause induction of maternal and fetal toxicity in mice during oral administration of up to 20 mg/kg/day (approximately 0, 5 times the corresponding dose in humans [MRHD] of diclofenac tablets, tablets, 200 mg/mg/gm. denec, body surface (BSA)) Based on is still in rats and rabbits at oral doses of up to 10 mg/kg/day (is about 0, 5, 1 hr. Corresponding human doses, based on BSA concordance, MRHD). In studies where pregnant rats received 2 or 4 mg/kg diclofenac (0, 1, 0, 2x) significant maternal toxicity (peritoneal inflammation, mortality) was observed from day 15 of gestation to day 21 of lactation. These maternal toxic doses were associated with dystocia, prolonged gestation, decreased fetal weight and lift, and decreased fetal survival. Diclofenac has been shown to pass placental signs in mice, rats, and people.
Birth.
The effects of diclofenac potassium tablets on family members have not been studied. Animal studies have shown that NSAIDs, including diclofenac prostaglandin synthesis is inhibited, causing family retention and increasing the incidence of stillbirths.
latent mothers.
Summary of risks based on available data, diclofenac may occur in breast milk. The benefits of breastfeeding for the baby’s development and well being must be weighed against the mother’s medical need for diclofenac potassium tablets and the potential for adverse effects of major maternal illnesses on the breastfed baby.
Data 1 Lady took orally a diclofenac Salt, 150 mg/day, washed down with milk. diclofenac A level of 100 µg/liter corresponds to an infant dose of 0.03 mg/kg/day. Diclofenac was not observed in the breast milk of 12 women diclofenac (or later oral 100 mg/day for 7 days, or a single intramuscular injection of 50 mg immediately after birth).
Pediatrics
Safety and efficacy in pediatric patients have not been determined.
Geriatric
Elderly patients are at greatest risk for adverse cardiovascular, gastrointestinal, and/or renal reactions associated with NSAID administration, compared to younger patients. If the anticipated benefit in elderly patients is greater than these risks, start way down the dosing spectrum and confirm with the patient the presence of adverse effects (warnings, gastrointestinal thrombosis, gastrointestinal bleeding, ulceration, and perforation, hepatotoxicity, nephrotoxicity and hyperkalemia, precautions, laboratory testing).
Diclofenac is largely eliminated by the kidneys, and it is known that the risk of adverse effects from this product may be higher in patients with reduced renal function. Elderly patients are advised to exercise caution in dose selection, as renal function decreases more frequently and it is healthier to keep renal function under control (see CLINICAL PHARMACOLOGY, Adverse Effects).
Side Effects
The following side effects are discussed in detail in other segments of this memo
- Cardiovascular thrombotic incidents (see WARNINGS)
- Gastrointestinal bleeding, ulceration, perforation (see WARNINGS)
- Hepatotoxicity (see WARNINGS)
- Hypertension (see WARNINGS)
- Heart deficiency and edema (see warnings)
- Kidney nephropathy and hyperkalemia (see WARNINGS)
- Anaphylactic reactions (see warnings)
- Severe skin reactions (see warnings)
- Hematologic Toxicity (see WARNINGS)
Clinical Research Experience
Because clinical studies are conducted under very different circumstances, the characteristics of side effects observed in a clinical study of a product may not be directly comparable to those observed in a clinical study of another product, and what they do not reflect are the actual observed characteristics.
718 patients treated with diclofenac tablets with a specific release for a short period of time, i.e., 2 months or less, were reported with side effects in one tenth to one tenth of the patients, as were those treated for a longer period. In a 6-month double-blind study comparing Voltren (n = 197) to ibuprofen (n = 197) opposite diclofenac potassium tablets (n = 196), side effects were comparable in nature and frequency.
In patients treated with diclofenac potassium tablets or other NSAIDs, adverse effects were reported more frequently, occurring in approximately 1% to 10% of patients.
Gastrointestinal problems, including abdominal pain, constipation, diarrhea, indigestion, flatulence, bleeding/perforation, heartburn, nausea, gastric ulcers, nausea.
Abnormal renal function, anemia, dizziness, edema, increased liver enzymes, headache, increased bleeding time, teeth, rash, ear rashes.
Additional side effects are occasional.
General: fever, infection, centichemia.
Cardiovascular: congestive heart failure, hypertension, tachycardia, syncope.
Gastrointestinal: dry mouth, esophageal infection, stomach/gastrointestinal
Hematologic and lymphatic: mottling, eosinophils, leukemia, melena, purpura, rectal bleeding, stomatitis, thrombocytopenia
Metabolism and nutrition: weight changes
Nervous system: fear, asthma, confusion, depression, abnormal dreams, drowsiness, insomnia, malaise, stress, paralysis, drowsiness, tremor, dizziness of respiratory system: asthma, dyspnuu
Skin and appendages: alopecia, light sensitivity, perspiration
Special senses: blurred vision
Urogenital system: cystitis, dysuria, hematuria, interstitial nephritis, oliguria/polyuria, proteinuria, renal failure
Other side effects occasionally observed:
General physical: anaphylactic reactions, loss of appetite, death
Cardiovascular: cardiac rhythm disturbances, hypotension, myocardial infarction, heart palpitations, vasculitis.
Gastrointestinal: colitis, burping, hepatitis with yellow und, parturition, liver necrosis, pancreatitis
Hemo- and lymphoid: agrocytosis, hemolytic anemia, nonplasmatic anemia, lymph node tumors, pancytopenia
Metabolism and saturation: hyperglycemia
Nervous system: cases, com sleep, hallucinations, meningitis
Respiratory: respiratory depression, pneumonia
Skin and Appendix: angioedema, toxic epidermal necrosis, erythema multiforme, exfoliative dermatitis, Stevens-Johnson syndrome, ur measles
Special senses: agitated inflammation, hearing loss
Overdose
Symptoms after acute administration of NSAID are generally limited to lethargy, drowsiness, nausea, vomiting, and annoying pain on the abdominal side, which usually helps. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, com sleep occurred, but was rare.
Treats symptomatic and supportive care patients after NSAID overdose. No special antidoter. Advise to osmotic catharsis (5-10 more appropriate doses) in symptomatic patients and massive overdose patients with vomiting and/or activated cabbage (60-100 grams in adult patients, 1-2 grams per kg body weight in pediatric patients) and/or observed after 4 hours. Forced diuresis, urinary worms, hemodialysis, or hemoperfusion may be unnecessary due to high protein binding.
For more information on overdose treatment, contact an addiction center at 1-800-222-1222.
Medication and Administration
Before making a decision about the use of diclofenac potassium tablets, carefully consider the superior properties and risks of diclofenac specific release tablets and other medications. Use the lowest effective dose for the shortest time according to the individual patient’s treatment goals (see Warnings, Gastrointestinal Bleeding, Ulcers, and Perforation).
After observing the response to initial therapy with diclofenac potassium tablets, the dose and frequency should be adapted to the needs of the individual patient.
For treatment of pain or primary dysmenorrhea, the correct dose is 50 mg three times daily. With skill, a physician can conclude that in some patients, an initial dose of 100 mg of diclofenac potassium tablets and a dose of 50 mg gives the best illumination.
For osteoarthritis lighting, the correct dose is 100-150 mg/day in divided doses, 50 mg twice a day or three times a day. or 3 times a day.
For relief of rheumatoid arthritis, the correct dose is 150-200 mg/day in divided doses, 50 mg 3 times a day or 4 times a day.
Various formulations of diclofenac [VOLTAREN ® ( diclofenac Sodium tablets with intestinal layer); Voltaren®-XR ( diclofenac sodium tablets with extended release); diclofenac potassium immediate-release pills] are not necessarily considered bioequivalent, even though the strength per milligram is the same.
How is diclofenac potassium delivered?
There is 50 mg of Diclofenac Potassium, USP available at USP.
Broken white to broken white to broken white, round, double-rooted 50 mg tablets, treated with “DP” on one side and “50” on the other.
NDC 70710-1832-1 With HDPE FLACON, 100 tablets with 100 tablets NDC 70710-1832-0
Store and pack in impenetrable, light resistant package as defined in the USP.
Store at room temperature 20°C to 25°C (68°F to 77°F). Expiration date allowed 15°C to 30°C (59°F to 86°F) [see Controlled Room Temperatures in USP]. Protect from moisture.
Medication guide available at www. zydususa. com/medguides or call 1-877-993-8779.
Manufactured by Umedica Laboratories Pvt. Ltd. site # 221, ND Phase II, GIDC, VAP I-396, 195, Gujarat, India.
Distributed by Zydus Pharmaceuticals (USA) Inc. Pennington, NJ 08534.
- Increased risk of heart attack or heart attack. This is fatal. This risk can occur at the start of treatment and may increase.
- Increased dosage of NSAIDs.
- Prolonged use of NSAIDs.
- Risk of bleeding, ulcers, and fissures (perforations) in the gastrointestinal tract (tube running from mouth to stomach), stomach, and intestinal tract:
- At any time during use.
- No warning symptoms.
- May cause death.
- History of stomach ulcers or stomach or gastrointestinal bleeding when using NSAIDs.
- Use of medications called “corticosteroids,” “anticoagulants,” “SSRIs,” or “SNRIS.”
- Increased doses of
- Longer use of NSAIDs
- Smoking
- Alcohol consumption
- Older age
- Weakness of disease
- Advanced liver disease
- Bleeding problems
- As prescribed
- At lowest dose for treatment
- Immediately
- Asthma attacks, hives, or other allergic reactions with aspirin or other NSAIDs.
- Immediately before or after heart bypass operation.
- Liver or kidney problems.
- I have high blood pressure.
- asthma
- You are pregnant or plan to become pregnant; taking an NSAID after about 20 months of pregnancy is harmful to the fetus. If you are 20-30 months pregnant and must take an NSAID for more than 2 days, your caregiver should track the amount of water in the uterus around the baby. After about 30 months of pregnancy, the NSAID no longer needs to be taken.
- You are breastfeeding or planning to breastfeed
- New or unhealthy high blood pressure
- Heart failure
- Liver problems, including liver failure
- Kidney problems, including renal failure
- Too few red blood cells (anemia)
- Life-threatening skin reactions
- Dangerous allergic reactions
- Other side effects of NSAIDs are stomach pain, constipation, diarrhea, flatulence, heartburn, lightheadedness, nausea, dizziness
- Shortness of breath or breathing problems
- Chest pain
- Weakness on one or both sides of your body
- Unclear speech
- Swelling of your face or throat
- Nausea
- Restless or weaker than normal
- Diarrhea
- Itching
- Your skin or eyes look yellow
- Stomach complaints or abdominal pain
- Flu symptoms
- Vomiting or diarrhea
- Is there blood in your intestines or is it dark and sticky?
- Abnormal weight gain
- Skin rash or blisters with fever
- Swelling of arms and legs, hands and feet
These are not all possible side effects of NSAIDs. Ask your doctor or pharmacist about NSAIDs for more information.
- Aspirin is an NSAID, but it does not increase the chance of heart attack. Aspirin can cause bleeding in the brain, stomach, and intestinal tract. Aspirin can cause stomach and intestinal ulcers.
- Some NSAIDs are sold at low doses without a prescription (over the counter); consult your own health care provider before using an NSAID without a prescription for more than 10 days.
Medication guide available at www. zydususa. com/medguides or call 1-877-993-8779.
Umedica Laboratories Pvt. Ltd.
Schedule No. 221, ND Phase II, GIDC, VAP I-396. 195,
Zydus Pharmaceuticals (USA) Inc.
Pennington, NJ 08534.
This medication instruction has been approved by the U.S. Food and Drug Administration.
Label Bag.
HDPE bottle of 100 tablets

HDPE Bottle of 1000 Tablets

Product Information Type. Drug labels for human prescriptions Dust Code (Source) NDC: 70710-1832 Dosing Method Oral DEA Schedule Active Component/ Active Ingredient Name Component Strength based on Courage Diclofenac potassium (diclofenac) Diclofenac Potassium 50 mg Inactive Ingredients Name Component Courage Mammary stain Microcrystalline cellulose Sodium lauryl sulfate Silicon dioxide Magnesium stearate Hypromellose Talc Titanium dioxide Product Properties Color White (broken white to almost white) Score No score Shape crotch Size 8 mm Taste Output data code DP; 50 Includes Package # Product Code Packaging Description 1 NDC: 70710-1832-1 100 film – coated tablets in 1 bottle 2 NDC: 70710-1832-0 1000 Films – Coated Tablet in 1 Bottle Marketing Information Marketing Category Order number or hyperlink to monograph Get out of start start Set end date And a ANDA215750 12. 05. 2022 Labelle r-Zydus Pharmaceuticals (USA) Inc.(156861945) Institution Name Address ID Card/FEI Activity Umedica Laboratories Privateit Limited 920635096 прои Neic-Indervers (70710-1832), аналиitting (70710-1832) Zydus Pharmaceuticals (USA) Inc.
Frequently Asked Question.
- Which anesthetic should I use for my feet?
- Why is diclofenac Is ibuprofen available only by prescription?
- Can I take ibuprofen at the same time as my blood pressure medication?
- Can an NSAID be used to cure fever caused by Covid-19?
- Does the drug diclofenac Are the medications related?
More about diclofenac
- Check for interactions
- Compare Alternatives
- Prices and Coupons
- Reviews (671)
- Medication Photos
- Recent Warnings FDA (11)
- Side Effects
- Dosing Information
- Tips for Patients
- Pregnancy
- Support Groups
- Product class: nonsteroidal anti-inflammatory drugs.
- Breastfeeding
- In Spanish
Patient Sources
Tools for Professionals
- Prescription
- diclofenac (FDA)
- diclofenac capsules (FDA)
- diclofenac tablets with delayed release (FDA)
- Diclofenac, tablet with extended release, 100 mg (FDA)
Other Brands
Related Treatments
- Ice shoulder
- Low Back Pain
- Ankylosing spondylitis
- Aseptic Necrosis
Diclofenac Potassium: Uses, Side Effects, and Almost Everything Else
Nonsteroidal anti-inflammatory drugs (including. diclofenac ) It can occasionally increase the risk of heart attack or heart attack. This effect can occur at any time during the use of this product, but is possible with long-term use. The risk may be increased in the elderly or in the presence of heart disease or increased risk of cardiovascular disease (e.g., smoking, family history of heart disease, or disorders such as high blood pressure or diabetes). do not take this product before or after Heartby Pass Operation (CABG).
Additionally, in rare cases, this product can cause substantial (rarely fatal) bleeding from the stomach or intestinal tract. This effect may occur without warning signal at any time while using this product. Elderly persons are at increased risk of this effect.
Stop taking diclofenac And if you notice one of these rare but nonsense side effects, seek medical help immediately: abdominal/stomach pain that does not pass, black/tricky stools, nausea that looks like coffee pigeons, chest/chin/pain in left arm, shortness of breath, unusual sweating, lost, impotence on one side of body, difficulty consulting , unexpected changes in vision.
Consult your own physician or pharmacist about the advantages and risks of using this product.
Warning:
Nonsteroidal anti-inflammatory drugs (including. diclofenac ) It can occasionally increase the risk of heart attack or heart attack. This effect can occur at any time during the use of this product, but is possible with long-term use. The risk may be increased in the elderly or in the presence of heart disease or increased risk of cardiovascular disease (e.g., smoking, family history of heart disease, or disorders such as high blood pressure or diabetes). do not take this product before or after Heartby Pass Operation (CABG).
Additionally, in rare cases, this product can cause substantial (rarely fatal) bleeding from the stomach or intestinal tract. This effect may occur without warning signal at any time while using this product. Elderly persons are at increased risk of this effect.
Stop taking diclofenac And if you notice one of these rare but nonsense side effects, seek medical help immediately: abdominal/stomach pain that does not pass, black/tricky stools, nausea that looks like coffee pigeons, chest/chin/pain in left arm, shortness of breath, unusual sweating, lost, impotence on one side of body, difficulty consulting , unexpected changes in vision.
Consult your own physician or pharmacist about the advantages and risks of using this product.
Instructions for Use
See more warnings. Some brands of this product may still reduce pain, swelling, and stiffness in arthritic joints. Reducing these symptoms can help you perform more of your normal daily activities. Diclofenac is a popular non-steroidal anti-inflammatory (NSAID). It blocks the body’s production of certain natural drugs that cause inflammation. This effect helps reduce swelling, pain, and fever. If you are treating a chronic condition such as arthritis, ask your doctor for a non-drug or use other medications to treat your pain.
How to use diclofenac potassium oral
Read your pharmacist’s drug instructions before you take them diclofenac And every time you get a refill. If you have questions, ask your doctor or pharmacist.
Take this medication by mouth as prescribed by your doctor, usually 2-4 times a day. Take this drink with a full glass of water (8 ounces/240 milliliters) unless your doctor tells you something else. Do not drink for more than 10 minutes after taking this medicine. If your stomach upsets while taking this medication, take it with food, milk, or a stomach acid inhibitor.
There are different brands and forms of this medication. Different forms do not have the same effect on the same effect and therefore the form cannot be changed. of diclofenac Unless your doctor tells you otherwise.
Dosage is based on welfare status, healing and response to other medications. To reduce the risk of stomach bleeding and other side effects, take this medication at the lowest dose in the shortest amount of time. Do not increase your own dosage. Also, do not take this medication for longer than specified. Talk to your doctor or pharmacist about the risks and excellence.
Certain disorders (such as arthritis) may take up to 2 months before you benefit from this product.
Remember that this product is more effective than anything else if used on an “as needed” basis (not on a simple schedule) and at the first symptoms of pain. If you wait until your pain worsens, the medication may not activate.
Tell your own doctor if your situation worsens.
Side Effects
See also the Warnings section.
Stomach complaints, nausea, heartburn, diarrhea, obstruction, flatulence, headache, drowsiness, dizziness, or blurred vision. If one of these effects persists or worsens, tell your doctor or pharmacist quickly.
Remember that this drug is prescribed because your doctor has determined that the benefit for you is greater than the risk of side effects. Almost everyone who uses this medication has no serious side effects.
This medication can raise your blood pressure. Check your blood pressure regularly and let your doctor know if the results are high.
Tell your doctor immediately if you have any serious side effects such as hearing changes (e.g. ringing in the ears), mental/mood changes, unusual fatigue, unusual/fast weight gain), creepy bleeding, difficult/painful swallowing, signs of heart deficiency (e.g. swollen ankles/feet).
If you have symptoms of kidney problems (e.g., changes in urine count, pink/bloody urine), inexplicable stiff necks, or other fairly serious side effects, find medical assistance immediately.
Diclofenac can sometimes cause non-energetic (possibly fatal) liver disease. Seek medical assistance immediately if there are signs of liver damage.
Very responsible allergic reactions to this product are rare. Nevertheless, seek medical assistance immediately if you notice signs of a severe allergic reaction, such as fever, swollen lymph nodes, skin rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, or bad breath.
This is not an absolute list of possible adverse reactions. If you notice any other effects not mentioned above, contact your physician or pharmacist.
In the U.S., call your doctor for medical advice regarding side effects at 1-800-FDA-1088 or www. You can report side effects to the FDA at FDA.FDA.Gov/Medwatch.
For medical advice on side effects, call your Canadian physician at 1-866-234-2345 to report side effects to Health Canada.
Precautions
See also the Warnings section.
Before taking diclofenac Tell your doctor or pharmacist if you are allergic to this medicine. or aspirin or another NSAID (such as ibuprofen, naproxen, celecoxib); or if you have other allergies. This product contains inactive ingredients that may cause allergic reactions or other problems. Consult your pharmacist for more information.
In particular, inform your doctor or pharmacist about your medical situation before using this medication: asthma (including situations where breathing shifts to the worst side after using aspirin or other NSAIDs), blood disorders (anemia, bleeding/clotting problems, etc.) , nasal congestion. (nasal polyps), heart problems (e.g., previous heart attack), high blood pressure, liver problems, heart attack, edema (swelling, fluid retention), stomach/intestinal/esophageal problems (bleeding, heartburn, stomach ulcers).
Kidney problems may present an opportunity to introduce NSAID medications. diclofenac Dehydration, older heart or kidney disease, or use of certain medications or use of certain medications increases the likelihood of kidney problems (see more interactions between medications). Drink plenty of water as prescribed by your doctor to prevent dehydration and tell your doctor immediately if you have pink/bloody urine or any unusual changes in your urine count.
This product may cause dizziness, drowsiness, or blurred vision. Alcohol or marijuana (cannabis) may exacerbate these effects. Do not ride, use machinery, or do anything that requires attention or clear view. Limit alcoholic beverages. Consult a physician if using marijuana (cannabis).
This drug can cause stomach bleeding. Daily use of alcohol and tobacco, especially in combination with this medication, may increase the risk of stomach bleeding. Limit alcohol use and stop smoking.
This medication can make you more sensitive to the sun. Limit time in the sun. Beware of tanning beds and lamps. Use sunscreen and wear protective clothing outdoors. Tell your own doctor immediately if you burn or blister/redness burns on your skin.
Tell your doctor or dentist about all products you use (including prescription, non-prescription resources, and herbal products).
The elderly are at greatest risk for stomach/intestinal bleeding, kidney problems, and heart attack when using this medication.
Before using this medication, women of fertile age should talk to their doctor about the benefits and risks. Tell your doctor if you are pregnant or plan to become pregnant. This drug is harmful to the fetus and can cause difficulties during childbirth. It is not recommended for use during the first 20 months of pregnancy for family members. If your doctor determines that you need to use this medication between 20 and 30 months of pregnancy, you should use the lowest effective dose during the shortest time. After 30 months of pregnancy, it is no longer necessary to use this drug.
This medication is converted to breast milk. Consult your physician before breastfeeding.






